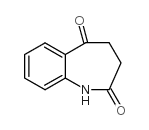
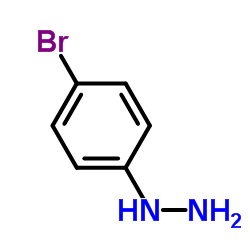
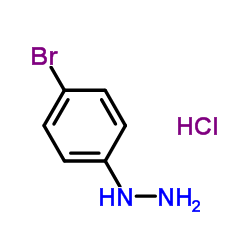
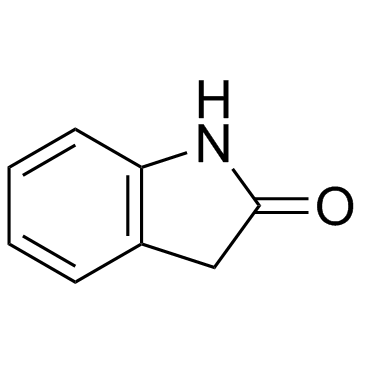
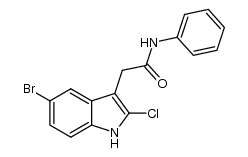
142273-20-9
| Name | Kenpaullone |
|---|---|
| Synonyms |
9-Bromo-7,12-dihydroindolo[3,2-d][1]benzazepin-6(5H)-one
9-bromo-7,12-dihydro-5H-indolo[3,2-d][1]benzazepin-6-one Kenpaullone MFCD02683595 9-Bromo-7,12-dihydro-indolo[3,2-d][1]benzazepin-6(5H)-one NSC-664704 |
| Description | Kenpaullone is a potent inhibitor of CDK1/cyclin B and GSK-3β, with IC50s of 0.4 μM and 23 nM, and also inhibits CDK2/cyclin A, CDK2/cyclin E, and CDK5/p25 with IC50s of 0.68 μM, 7.5 μM, 0.85 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Cdk1/cyclin B:0.4 μM (IC50) cdk2/cyclin A:0.68 μM (IC50) CDK5/p35:0.85 μM (IC50) CDK2/cyclinE:7.5 μM (IC50) GSK-3β:0.023 μM (IC50) erk1:20 μM (IC50) erk2:9 μM (IC50) c-raf:38 μM (IC50) |
| In Vitro | Kenpaullone shows much less effect on c-src (IC50, 15 μM), casein kinase 2 (IC50, 20 μM), erk 1 (IC50, 20 μM), and erk 2 (IC50, 9 μM). Kenpaullone acts by competitive inhibition of ATP binding, and the apparent Ki is 2.5 μM. Kenpaullone can inhibit the growth of tumor cells in culture (mean GI50, 43 μM) and causes altered cell cycle progression most clearly revealed under conditions of recovery from serum starvation[1]. Kenpaullone demonstrates a wide range of biological utility, extending from maintenance of pancreatic β cell survival and proliferation to the induction of apoptosis in cancer cells[2]. |
| Kinase Assay | The kinase assay is run for 10 min at 30°C with 1 mg/mL histone H1, in the presence of 15 μM [g-32P]ATP (3000 Ci/μmol; 1 mCi/mL) in a final volume of 30 ml. Purification and assays or inhibition of other kinases are performed. In kinetic experiments, the histone H1 concentration is lowered to 3.5 mg/mL; the ATP concentration ranged from 50 to 400 μM, and the kenpaullone concentration ranges from 1 to 4 μM. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 613.0±45.0 °C at 760 mmHg |
| Melting Point | >300ºC (dec.) |
| Molecular Formula | C16H11BrN2O |
| Molecular Weight | 327.175 |
| Flash Point | 324.5±28.7 °C |
| Exact Mass | 326.005463 |
| PSA | 44.89000 |
| LogP | 4.02 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.730 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 18 mg/mL, clear, yellow |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |