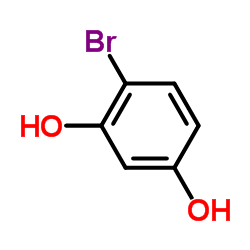
479-13-0
| Name | coumestrol |
|---|---|
| Synonyms |
3,9-Dihydroxycoumestan
EINECS 207-525-6 Cumoesterol Cumoestrol MFCD00016885 7',6-Dihydroxycoumarino(3',4',3,2)coumarone 3,9-dihydroxy-[1]benzofuro[3,2-c]chromen-6-one Coumestrol Cumostrol 3,9-Dihydroxy-6H-benzofuro[3,2-c][1]benzopyran-6-one 7,12-Dihydroxycoumestan coumesterol 3,9-Dihydroxy-6H-[1]benzofuro[3,2-c]chromen-6-one 2-(2,4-Dihydroxyphenyl)-6-hydroxy-3-benzofurancarboxylic Acid d-Lactone |
| Description | Coumestrol, a phytoestrogen present in soybean products, exhibits activities against cancers, neurological disorders, and autoimmune diseases. It suppresses proliferation of ES2 cells with an IC50 of 50 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 50 μM[1] |
| In Vitro | Coumestrol exerts chemotherapeutic effects via PI3K and ERK1/2 MAPK pathways. Coumestrol inhibits viability and invasion, and induces apoptosis of ES2 (clear cell-/serous carcinoma origin) cells. In addition, immunoreactive PCNA and ERBB2, markers of proliferation of ovarian carcinoma, are attenuated in their expression in coumestrol-induced death of ES2 cells. Phosphorylation of AKT, p70S6K, ERK1/2, JNK1/2 and p90RSK is inactivated by coumestrol treatment in a dose- and time-dependent manner[1]. Coumestrol inhibits proliferation and induces apoptosis in MCF-7 cells, which is prevented by copper chelator neocuproine and ROS scavengers. Coumestrol treatment induces ROS generation coupled to DNA fragmentation, up-regulation of p53/p21, cell cycle arrest at G1/S phase, mitochondrial membrane depolarization and caspases 9/3 activation[2]. |
| Cell Assay | To determine dose-dependent effects of coumestrol, ES2 cells are treated with different concentrations (0, 1, 10, 20, 50 or 100 μM) of coumestrol[1]. Coumestrol is dissolved in DMSO to prepare a 3 mM stock. Breast cancer MCF-7 cells are treated with increasing concentrations of coumestrol for 24, 48 and 72 h. Then, 20 μL of MTT (5 mg/mL) is added each well and re-incubated for additional 3 h. Formazan blue crystals formed are dissolved in 100 μL of DMSO. Absorbance is read at 570 nm using ELISA plate reader[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 406.0±24.0 °C at 760 mmHg |
| Melting Point | ≥350ºC(lit.) |
| Molecular Formula | C15H8O5 |
| Molecular Weight | 268.221 |
| Flash Point | 199.3±22.9 °C |
| Exact Mass | 268.037170 |
| PSA | 83.81000 |
| LogP | 2.94 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.768 |
| Storage condition | Refrigerator |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R22 |
| Safety Phrases | 26-36 |
| WGK Germany | 3 |
| RTECS | DF8077000 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |


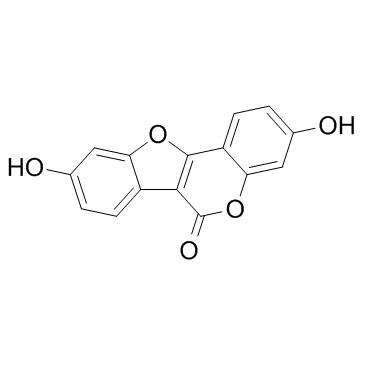
![3-hydroxy-6-oxo-6H-benzofuro[3,2-c]chromen-9-yl 4-methylbenzenesulfonate structure](https://image.chemsrc.com/caspic/127/870456-46-5.png)
![2-(2,4-diacetoxyphenyl)-3-iodo-6-(tosyloxy)benzo[b]furan structure](https://image.chemsrc.com/caspic/165/870456-45-4.png)

![Methyl 6-hydroxy-2-[2-hydroxy-4-(tosyloxy)phenyl]benzo[b]furan-3-carboxylate structure](https://image.chemsrc.com/caspic/170/328945-98-8.png)