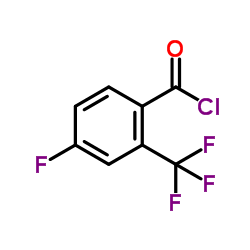
1258861-20-9
| Name | 4-fluoro-N-methyl-N-[1-[4-(2-methylpyrazol-3-yl)phthalazin-1-yl]piperidin-4-yl]-2-(trifluoromethyl)benzamide |
|---|---|
| Synonyms |
Taladegib
4-Fluoro-N-methyl-N-{1-[4-(1-methyl-1H-pyrazol-5-yl)-1-phthalazinyl]-4-piperidinyl}-2-(trifluoromethyl)benzamide LY 2940680 LY2940680 4-fluoro-N-methyl-N-(1-(4-(1-methyl-1H-pyrazol-5-yl)phthalazin-1-yl)piperidin-4-yl)-2-(trifluoromethyl)benzamide |
| Description | Taladegib (LY2940680) is an antagonist of the smoothened receptor. |
|---|---|
| Related Catalog | |
| Target |
Smo[1] |
| In Vitro | Taladegib, a small-molecule antagonist of the smoothened receptor, shows a slight inhibitory effect on cell proliferation without differences between mucin- (IC50: Taladegib=49.8±4.5 μM) and mixed- Cholangiocarcinoma (CCA) (IC50: Taladegib=61.2±21.1 μM)[1]. The IC50 for Taladegib inhibition of [3H]MRT-92 binding is right shifted (3- to 100-fold) for the S387AECL2, L325F3.36f, and D473H6.54f mutants but did not differ from that of WT receptor for the other mutants. The ability of SANT-1 to inhibit [3H]MRT-92 binding to V329F3.40f and T466F6.47f mutants is abolished, and it is severely impaired for L325F3.40f, I408F5.51f, and M525G7.45f mutants (4- to 140-fold drop of the IC50), but is not modified for the S387AECL2 mutant. Taken together, these data confirm our docking hypothesis that MRT-92-binding mode differs from that of either Taladegib or SANT-1 by simultaneously occupying binding sites 1 and 2[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 703.5±60.0 °C at 760 mmHg |
| Molecular Formula | C26H24F4N6O |
| Molecular Weight | 512.502 |
| Flash Point | 379.3±32.9 °C |
| Exact Mass | 512.194763 |
| PSA | 67.15000 |
| LogP | 2.70 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.634 |
| Storage condition | -20°C |
| Hazard Codes | Xi |
|---|
|
~83% 
1258861-20-9 |
| Literature: US2010/324048 A1, ; Page/Page column 6 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |