γ-Valerolactone

γ-Valerolactone structure
|
Common Name | γ-Valerolactone | ||
|---|---|---|---|---|
| CAS Number | 108-29-2 | Molecular Weight | 100.12 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 206.6±8.0 °C at 760 mmHg | |
| Molecular Formula | C5H8O2 | Melting Point | -31ºC | |
| MSDS | Chinese USA | Flash Point | 81.1±0.0 °C | |
Use of γ-Valerolactone5-Methyldihydrofuran-2(3H)-one is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | γ-valerolactone |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Methyldihydrofuran-2(3H)-one is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | γ-戊内酯是一种分析参考标准品,被归类为 γ-羟基戊酸的前药形式。1 本产品旨在用于研究和法医应用。 |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 206.6±8.0 °C at 760 mmHg |
| Melting Point | -31ºC |
| Molecular Formula | C5H8O2 |
| Molecular Weight | 100.12 |
| Flash Point | 81.1±0.0 °C |
| Exact Mass | 100.052429 |
| PSA | 26.30000 |
| LogP | -0.27 |
| Vapour density | 3.45 (vs air) |
| Vapour Pressure | 0.2±0.4 mmHg at 25°C |
| Index of Refraction | 1.431 |
| InChIKey | GAEKPEKOJKCEMS-UHFFFAOYSA-N |
| SMILES | CC1CCC(=O)O1 |
| Water Solubility | MISCIBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36 |
| Safety Phrases | S26-S39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | LU3580000 |
| Packaging Group | I; II; III |
| HS Code | 29322980 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932209090 |
|---|---|
| Summary | 2932209090. other lactones. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Isolation and characterization of rat intestinal bacteria involved in biotransformation of (-)-epigallocatechin.
Arch. Microbiol. 196(10) , 681-95, (2014) Two intestinal bacterial strains MT4s-5 and MT42 involved in the degradation of (-)-epigallocatechin (EGC) were isolated from rat feces. Strain MT4s-5 was tentatively identified as Adlercreutzia equol... |
|
|
Bioavailability and catabolism of green tea flavan-3-ols in humans
Nutrition 26(11-12) , 1110-6, (2010) Objective The aim of this study was to investigate green tea flavan-3-ol catabolism and plasma pharmacokinetic and urinary excretion by high-performance liquid chromatography with tandem mass spectrom... |
|
|
Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone.
Science 343(6168) , 277-80, (2014) Widespread production of biomass-derived fuels and chemicals will require cost-effective processes for breaking down cellulose and hemicellulose into their constituent sugars. Here, we report laborato... |
| γ-Valeryllactone |
| Pentanoic acid, 4-hydroxy-, .γ.-lactone |
| gamma-valerolactone |
| 2(3H)-Furanone, dihydro-5-methyl- |
| (±)-4-Methylbutyrolactone |
| 4-Valerolactone |
| Dihydro-5-methyl-2(3H)-furanone |
| 4,5-Dihydro-5-methyl-2(3H)-furanone |
| (±)-γ-Valerolactone |
| Valeric acid, 4-hydroxy-, γ-lactone |
| EINECS 203-569-5 |
| Valerolactone |
| Pentanoic acid, 4-hydroxy-, γ-lactone |
| 5-methyldihydrofuran-2(3H)-one |
| 2(3H)-Furanone, dihydro-5-methyl-, (±)- |
| 5-Methyldihydro-2(3H)-furanone |
| furan-2-one, 5-methyltetrahydro- |
| dihydro-5-methyl-2(3H)-furanone, |
| γ-Valerolactone |
| MFCD00005400 |
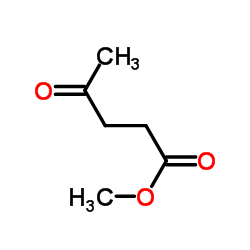 CAS#:624-45-3
CAS#:624-45-3 CAS#:539-88-8
CAS#:539-88-8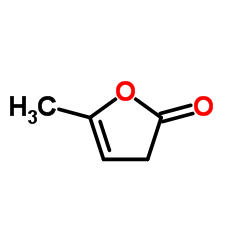 CAS#:591-12-8
CAS#:591-12-8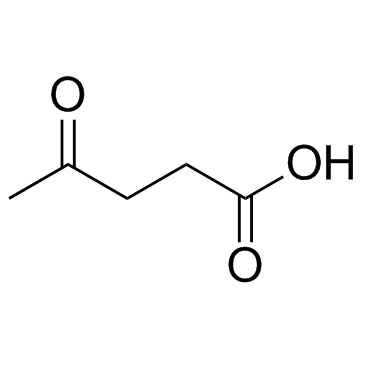 CAS#:123-76-2
CAS#:123-76-2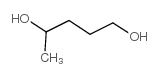 CAS#:626-95-9
CAS#:626-95-9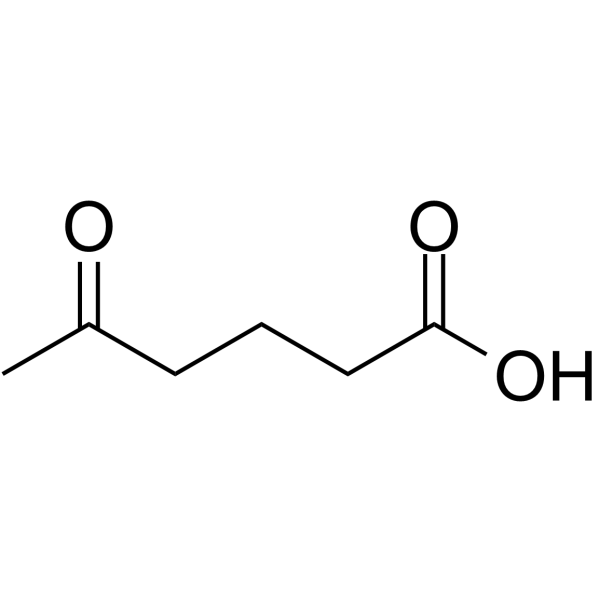 CAS#:3128-06-1
CAS#:3128-06-1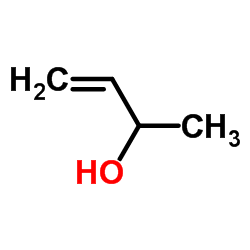 CAS#:598-32-3
CAS#:598-32-3 CAS#:201230-82-2
CAS#:201230-82-2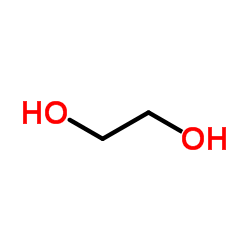 CAS#:107-21-1
CAS#:107-21-1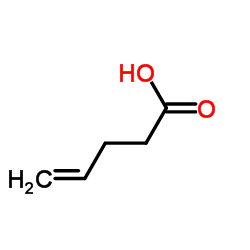 CAS#:591-80-0
CAS#:591-80-0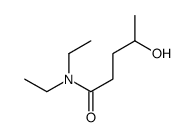 CAS#:112146-12-0
CAS#:112146-12-0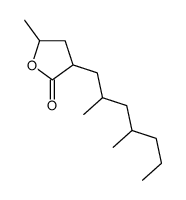 CAS#:110444-32-1
CAS#:110444-32-1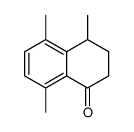 CAS#:10468-61-8
CAS#:10468-61-8 CAS#:502-56-7
CAS#:502-56-7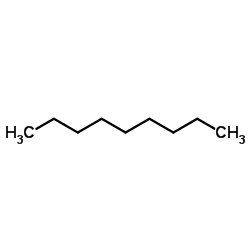 CAS#:111-84-2
CAS#:111-84-2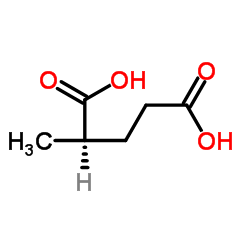 CAS#:18069-17-5
CAS#:18069-17-5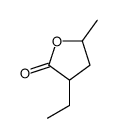 CAS#:19639-00-0
CAS#:19639-00-0 CAS#:2167-39-7
CAS#:2167-39-7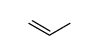 CAS#:187737-37-7
CAS#:187737-37-7 CAS#:54509-73-8
CAS#:54509-73-8
