allylthiourea

allylthiourea structure
|
Common Name | allylthiourea | ||
|---|---|---|---|---|
| CAS Number | 109-57-9 | Molecular Weight | 116.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 191.3±33.0 °C at 760 mmHg | |
| Molecular Formula | C4H8N2S | Melting Point | 70-72 °C(lit.) | |
| MSDS | USA | Flash Point | 69.5±25.4 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of allylthioureaAllylthiourea is a metabolic inhibitor that selective inhibits ammonia oxidation.Target: OthersAllylthiourea selectively inhibits ammonia oxidation at concentrations 8-80 μM. Allylthiourea (1 μM)inhibits ammonia oxidation by 80%. Complete inhibition is observed at an Allylthiourea concentration of 86 μM [1]. The inhibition of Allylthiourea on ammonia oxidation probably acts through chelating the copper of the ammonia monooxygenase active site. Allylthiourea is able to produce soluble methane monooxygenase (sMMO) in the presence of copper. Addition of 25 μM Allylthiourea decreases intracellular copper by 48% in Methylosinus trichosporium OB3b, allowing sMMO production at Cu/biomass ratios normally not permitting sMMO synthesis, which achieves a plateau of 320 μmol naphthol formed per gram dry biomass per hour [2]. |
| Name | Allylthiourea |
|---|---|
| Synonym | More Synonyms |
| Description | Allylthiourea is a metabolic inhibitor that selective inhibits ammonia oxidation.Target: OthersAllylthiourea selectively inhibits ammonia oxidation at concentrations 8-80 μM. Allylthiourea (1 μM)inhibits ammonia oxidation by 80%. Complete inhibition is observed at an Allylthiourea concentration of 86 μM [1]. The inhibition of Allylthiourea on ammonia oxidation probably acts through chelating the copper of the ammonia monooxygenase active site. Allylthiourea is able to produce soluble methane monooxygenase (sMMO) in the presence of copper. Addition of 25 μM Allylthiourea decreases intracellular copper by 48% in Methylosinus trichosporium OB3b, allowing sMMO production at Cu/biomass ratios normally not permitting sMMO synthesis, which achieves a plateau of 320 μmol naphthol formed per gram dry biomass per hour [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 191.3±33.0 °C at 760 mmHg |
| Melting Point | 70-72 °C(lit.) |
| Molecular Formula | C4H8N2S |
| Molecular Weight | 116.185 |
| Flash Point | 69.5±25.4 °C |
| Exact Mass | 116.040817 |
| PSA | 70.14000 |
| LogP | 0.12 |
| Vapour Pressure | 0.5±0.4 mmHg at 25°C |
| Index of Refraction | 1.562 |
| InChIKey | HTKFORQRBXIQHD-UHFFFAOYSA-N |
| SMILES | C=CCNC(N)=S |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | 67 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45-S24/25 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | YR8050000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 29309070 |
| Precursor 6 | |
|---|---|
| DownStream 9 | |
| HS Code | 29309070 |
|---|
|
Why Small Differences Matter: Elucidation of the Mechanisms Underlying the Transformation of 2OH- and 3OH-Carbamazepine in Contact with Sand Filter Material.
Environ. Sci. Technol. 49 , 10449-56, (2015) Carbamazepine (CBZ) is a worldwide used antiepileptic drug, which is metabolized to a large extent in the human body to several metabolites, including 10,11-dihydroxy-10,11-dihydrocarbamazepine (DiOHC... |
|
|
Construction of a highly bioluminescent Nitrosomonas as a probe for nitrification conditions.
Arch. Microbiol. 172(1) , 45-50, (1999) Cloned luciferase-encoding operons were transferred by conjugation to a natural isolate of the ammonia-oxidizing bacterial strain Nitrosomonas sp. RST41-3, thereby establishing conjugation as a tool f... |
|
|
The effect of thiosulphate and other inhibitors of autotrophic nitrification on heterotrophic nitrifiers.
Antonie van Leeuwenhoek 56(4) , 301-9, (1989) It has been found that heterotrophic nitrification by Thiosphaera pantotropha can be inhibited by thiosulphate in batch and chemostat cultures. Allythiourea and nitrapyrin, both classically considered... |
| 1-Prop-2-en-1-ylthioharnstoff |
| prop-2-enylthiourea |
| Allyl thiourea |
| Thiosinamine |
| 1-Allyl-2-thiourea |
| EINECS 203-683-5 |
| thiourea, N-2-propenyl- |
| 1-Allylthiourea |
| Thiourea, N-2-propen-1-yl- |
| MFCD00004940 |
| allylthiourea |
 CAS#:24966-88-9
CAS#:24966-88-9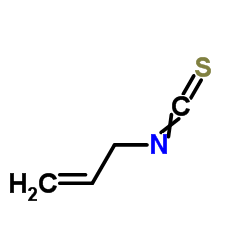 CAS#:57-06-7
CAS#:57-06-7 CAS#:557-11-9
CAS#:557-11-9 CAS#:67917-68-4
CAS#:67917-68-4 CAS#:862-64-6
CAS#:862-64-6 CAS#:7664-41-7
CAS#:7664-41-7 CAS#:111915-74-3
CAS#:111915-74-3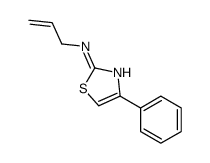 CAS#:21344-73-0
CAS#:21344-73-0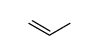 CAS#:187737-37-7
CAS#:187737-37-7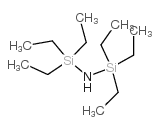 CAS#:2117-18-2
CAS#:2117-18-2 CAS#:994-49-0
CAS#:994-49-0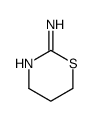 CAS#:30480-64-9
CAS#:30480-64-9 CAS#:10416-80-5
CAS#:10416-80-5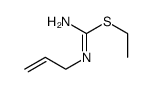 CAS#:100139-39-7
CAS#:100139-39-7
