TM 5275 sodium salt
Modify Date: 2025-08-25 19:06:45
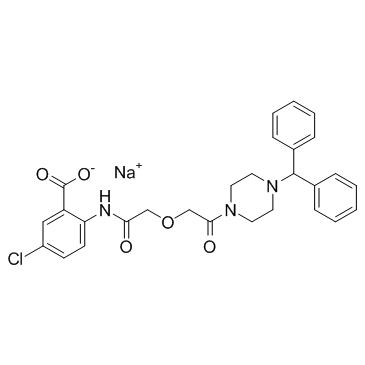
TM 5275 sodium salt structure
|
Common Name | TM 5275 sodium salt | ||
|---|---|---|---|---|
| CAS Number | 1103926-82-4 | Molecular Weight | 544.98 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C28H28ClN3NaO5 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of TM 5275 sodium saltTM5275 sodium is a plasminogen activator inhibitor (PAI-1) with an IC50 of 6.95 μM. |
| Name | sodium 5-chloro-2-[({2-[4-(diphenylmethyl)piperazin-1-yl]-2-oxoethoxy}acetyl)amino]benzoate |
|---|---|
| Synonym | More Synonyms |
| Description | TM5275 sodium is a plasminogen activator inhibitor (PAI-1) with an IC50 of 6.95 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 6.95 μM (PAI-1)[1] |
| In Vitro | Docking studies shows that TM5275 binds to strand 4 of the A β-sheet (s4A) position of PAI-1. TM5275 is a selective PAI-1 and (up to 100 μM) does not interfere with other serpin/serine protease systems[1]. TM5275 at concentrations of 20 and 100 μM significantly prolongs the retention of tPA-GFP on VECs by inhibiting tPA-GFP-PAI-1 high-molecular-weight complex formation. TM5275 enhances the time-dependent accumulation of plasminogen as well as the dissolution of fibrin clots on and around the tPA-GFP-expressing cells[2]. Cell viability at 72 h treatment is decreased with 70-100 μM TM5275 in ES-2 and JHOC-9 cells. From 48 h up to 96 h, cell growth is suppressed with 100 μM TM5275. Active PAI-1 in cell culture media is significantly decreased in cells treated with 100 μM TM5275 compared to control treatment. TM5275 is suggested to exert anti-proliferative effects in ovarian cancer with high PAI-1 expression[3]. |
| In Vivo | TM5275 exhibits a favorable pharmacokinetics profile and very low toxicity to mice and rats. In rat thrombosis models. Blood clot weights are significantly lower in rats administered 10 and 50 mg/kg of TM5275 (60.9±3.0 and 56.8±2.8 mg, respectively) than in vehicle-treated rats (72.5±2.0 mg). The antithrombotic effectiveness of TM5275 (50 mg/kg) is equivalent to that of ticlopidine (500 mg/kg), a reference antithrombotic compound. Plasma concentration of TM5275 reaches 17.5±5.2 μM after a dose of 10 mg/kg. TM5275 (5 mg/kg) combined with tPA (0.3 mg/kg) significantly enhances the antithrombotic effect of tPA (0.3 mg/kg) alone and provides a benefit similar to that of a high tPA dose (3 mg/kg)[1]. |
| Kinase Assay | TM5275 exhibits a favorable pharmacokinetics profile and very low toxicity to mice and rats. In rat thrombosis models. Blood clot weights are significantly lower in rats administered 10 and 50 mg/kg of TM5275 (60.9±3.0 and 56.8±2.8 mg, respectively) than in vehicle-treated rats (72.5±2.0 mg). The antithrombotic effectiveness of TM5275 (50 mg/kg) is equivalent to that of ticlopidine (500 mg/kg), a reference antithrombotic compound. Plasma concentration of TM5275 reaches 17.5±5.2 μM after a dose of 10 mg/kg. TM5275 (5 mg/kg) combined with tPA (0.3 mg/kg) significantly enhances the antithrombotic effect of tPA (0.3 mg/kg) alone and provides a benefit similar to that of a high tPA dose (3 mg/kg)[1]. |
| Cell Assay | ES2 cells are treated with DMSO (control) or 100 μM TM5275 for the indicated periods (24, 48, 72, 96 hour). Cell growth is determined by CellTiter-Glo assay[1]. |
| Animal Admin | Rats: Thrombus formation in arteriovenous shunts is achieved in male CD rats. Either TM5275 (10 and 50 mg/kg, n=9) or ticlopidine (500 mg/kg, n=6), suspended in 0.5% CMC solution, is administered orally by gavage 90 mins before the study. Control rats are administered only a 0.5% CMC solution (n=10). Blood is allowed to circulate through the shunt for 30 mins. The wet weight of the thrombus covering the silk thread is eventually measured[1]. Mice: TM5275 is administered orally by gavage to male ICR mice (50 mg/kg). Heparinized blood samples are collected from the vein before (0 h) and 1, 2, 6, and 24 h after oral drug administration. Plasma drug concentration is determined on a reverse-phase high-performance liquid chromatography[1]. |
| References |
| Molecular Formula | C28H28ClN3NaO5 |
|---|---|
| Molecular Weight | 544.98 |
| PSA | 102.01000 |
| LogP | 2.54120 |
| InChIKey | JSHSGBIWNPQCQZ-UHFFFAOYSA-M |
| SMILES | O=C(COCC(=O)N1CCN(C(c2ccccc2)c2ccccc2)CC1)Nc1ccc(Cl)cc1C(=O)[O-].[Na+] |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H400 |
| Precautionary Statements | P273-P301 + P312 + P330-P391 |
| RIDADR | UN 3077 9 / PGIII |
| sodium 2-(2-(2-(4-benzhydrylpiperazin-1-yl)-2-oxoethoxy)acetamido)-5-chlorobenzoate |
| TM5275 |
| Sodium 5-chloro-2-[({2-[4-(diphenylmethyl)-1-piperazinyl]-2-oxoethoxy}acetyl)amino]benzoate |
| Benzoic acid, 5-chloro-2-[[2-[2-[4-(diphenylmethyl)-1-piperazinyl]-2-oxoethoxy]acetyl]amino]-, sodium salt (1:1) |
| TM5275 sodium salt |
| TM5275 (sodium) |