CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
VS2830000
-
CHEMICAL NAME :
-
9,10-Secochola-5,7,10(19),22-tetraene-1,3,24-triol, 24-cyclopropyl-, (1-alpha,3-beta,5Z,7E,22E,24S)-
-
CAS REGISTRY NUMBER :
-
112965-21-6
-
LAST UPDATED :
-
199703
-
DATA ITEMS CITED :
-
11
-
MOLECULAR FORMULA :
-
C27-H40-O3
-
MOLECULAR WEIGHT :
-
412.67
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
10 gm/kg/6W-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 306,896,1993
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
11 gm/kg/11W-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 306,896,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>15 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - ataxia Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(Suppl 2),277,1996
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2190 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(Suppl 2),277,1996
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>1500 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - nausea or vomiting Musculoskeletal - other changes
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(Suppl 2),277,1996 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
560 ug/kg/4W-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - corneal damage Kidney, Ureter, Bladder - other changes in urine composition Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(Suppl 2),287,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
364 ug/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(Suppl 2),325,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
728 ug/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Skin and Appendages - dermatitis, other (after systemic exposure) Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(Suppl 2),365,1996 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
275 ug/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(suppl 2),403,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
650 ug/kg
-
SEX/DURATION :
-
female 17-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(suppl 2),439,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
13 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 21(suppl 2),425,1996
|
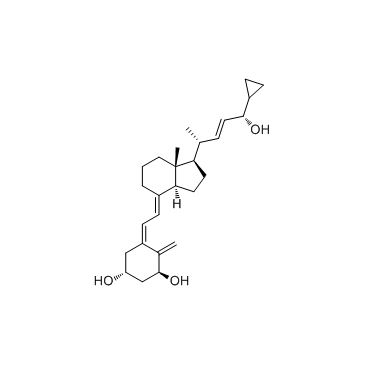
 CAS#:112875-61-3
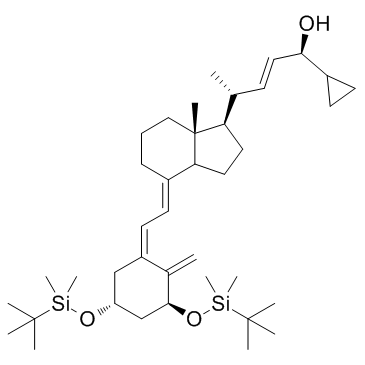
CAS#:112875-61-3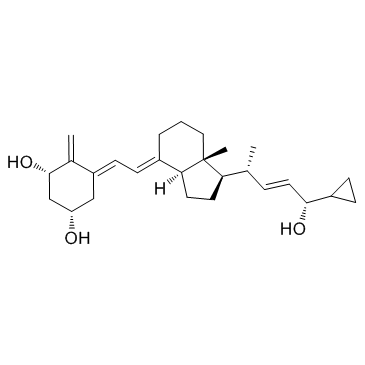 CAS#:113082-99-8
CAS#:113082-99-8 CAS#:765-43-5
CAS#:765-43-5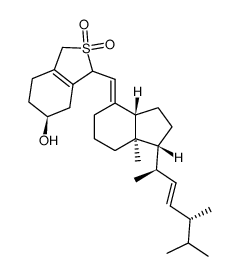 CAS#:87680-65-7
CAS#:87680-65-7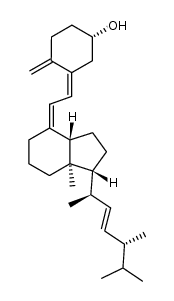 CAS#:247900-07-8
CAS#:247900-07-8 CAS#:590366-68-0
CAS#:590366-68-0![tert-butyl-[(1R,3S,5Z)-3-[tert-butyl(dimethyl)silyl]oxy-5-(2-diphenylphosphorylethylidene)-4-methylidenecyclohexyl]oxy-dimethylsilane Structure](https://image.chemsrc.com/caspic/143/81522-68-1.png) CAS#:81522-68-1
CAS#:81522-68-1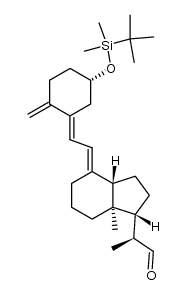 CAS#:112828-12-3
CAS#:112828-12-3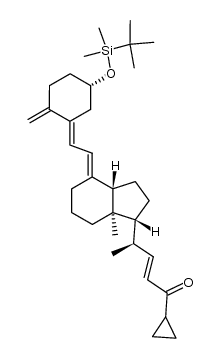 CAS#:112849-18-0
CAS#:112849-18-0 CAS#:87407-47-4
CAS#:87407-47-4
