Flufenamic acid-d4
Modify Date: 2025-08-27 18:16:47
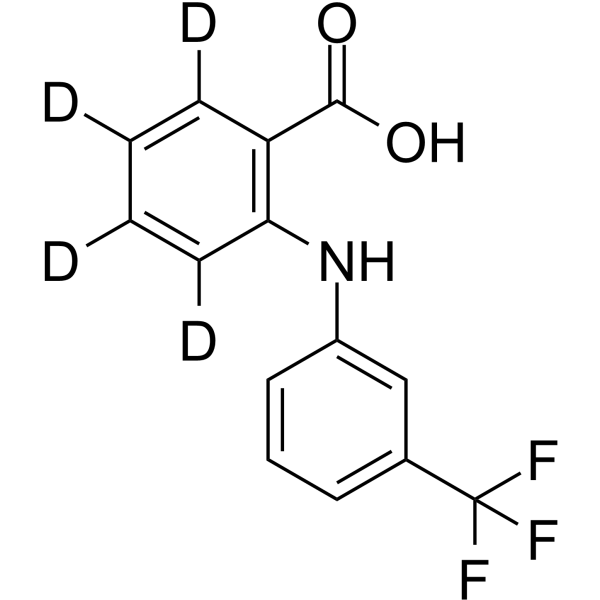
Flufenamic acid-d4 structure
|
Common Name | Flufenamic acid-d4 | ||
|---|---|---|---|---|
| CAS Number | 1185071-99-1 | Molecular Weight | 285.25 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H6D4F3NO2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Flufenamic acid-d4Flufenamic acid-d4 is deuterium labeled Flufenamic acid. Flufenamic acid is a non-steroidal anti-inflammatory agent, inhibits cyclooxygenase (COX), activates AMPK, and also modulates ion channels, blocking chloride channels and L-type Ca2+ channels, modulating non-selective cation channels (NSC), activating K+ channels. Flufenamic acid binds to the central pocket of TEAD2 YBD and inhibits both TEAD function and TEAD-YAP-dependent processes, such as cell migration and proliferation. |
| Name | Flufenamic acid-d4 |
|---|
| Description | Flufenamic acid-d4 is deuterium labeled Flufenamic acid. Flufenamic acid is a non-steroidal anti-inflammatory agent, inhibits cyclooxygenase (COX), activates AMPK, and also modulates ion channels, blocking chloride channels and L-type Ca2+ channels, modulating non-selective cation channels (NSC), activating K+ channels. Flufenamic acid binds to the central pocket of TEAD2 YBD and inhibits both TEAD function and TEAD-YAP-dependent processes, such as cell migration and proliferation. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C14H6D4F3NO2 |
|---|---|
| Molecular Weight | 285.25 |
| InChIKey | LPEPZBJOKDYZAD-ZEJCXXMWSA-N |
| SMILES | O=C(O)c1ccccc1Nc1cccc(C(F)(F)F)c1 |