Guanfacine-13C,15N3
Modify Date: 2025-08-24 22:58:46
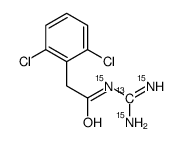
Guanfacine-13C,15N3 structure
|
Common Name | Guanfacine-13C,15N3 | ||
|---|---|---|---|---|
| CAS Number | 1189924-28-4 | Molecular Weight | 250.06600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C9H9Cl2N3O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Guanfacine-13C,15N3Guanfacine-13C,15N3 is the 13C and 15N labeled Guanfacine[1]. Guanfacine is an orally active noradrenergic α2A agonist and has high selective for the α2A receptor subtype. Guanfacine has effects in producing hypotension and sedation. Guanfacine can be used for the research of a variety of prefrontal cortex (PFC) cognitive disorders, including tourette's syndrome and attention deficit hyperactivity disorder (ADHD)[2][3][4]. |
| Name | N-[bis(azanyl)methylidene]-2-(2,6-dichlorophenyl)acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Guanfacine-13C,15N3 is the 13C and 15N labeled Guanfacine[1]. Guanfacine is an orally active noradrenergic α2A agonist and has high selective for the α2A receptor subtype. Guanfacine has effects in producing hypotension and sedation. Guanfacine can be used for the research of a variety of prefrontal cortex (PFC) cognitive disorders, including tourette's syndrome and attention deficit hyperactivity disorder (ADHD)[2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C9H9Cl2N3O |
|---|---|
| Molecular Weight | 250.06600 |
| Exact Mass | 249.00700 |
| PSA | 82.46000 |
| LogP | 3.18590 |
| Guarfacine-13C,15N3 |
| Guanfacine-13C,15N3 |
| Guanfacine0-13C,15N3 |
| Guanfascine-13C,15N3 |