Sulfabenzamide
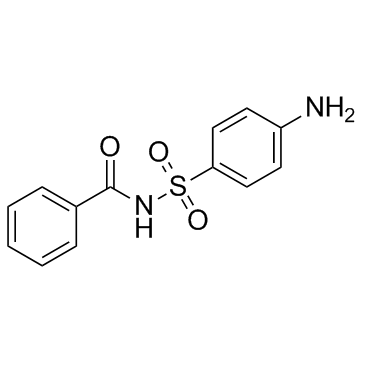
Sulfabenzamide structure
|
Common Name | Sulfabenzamide | ||
|---|---|---|---|---|
| CAS Number | 127-71-9 | Molecular Weight | 276.311 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C13H12N2O3S | Melting Point | 180-184 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of SulfabenzamideSulfabenzamide is a intermediate in the synthesis of organic and pharmaceutical. |
| Name | Sulfabenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | Sulfabenzamide is a intermediate in the synthesis of organic and pharmaceutical. |
|---|---|
| Related Catalog |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 180-184 °C |
| Molecular Formula | C13H12N2O3S |
| Molecular Weight | 276.311 |
| Exact Mass | 276.056854 |
| PSA | 97.64000 |
| LogP | 1.19 |
| Index of Refraction | 1.636 |
| InChIKey | PBCZLFBEBARBBI-UHFFFAOYSA-N |
| SMILES | Nc1ccc(S(=O)(=O)NC(=O)c2ccccc2)cc1 |
| Storage condition | 2-8°C |
| Water Solubility | 0.3 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | N |
| Safety Phrases | S22-S24/25 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | CV5802500 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2934999090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
|
|
A predictive ligand-based Bayesian model for human drug-induced liver injury.
Drug Metab. Dispos. 38 , 2302-8, (2010) Drug-induced liver injury (DILI) is one of the most important reasons for drug development failure at both preapproval and postapproval stages. There has been increased interest in developing predicti... |
| Benzamide, N-[(4-aminophenyl)sulfonyl]- |
| N-[(4-Aminophenyl)sulfonyl]benzamide |
| N-(4-Aminobenzenesulfonyl)benzamide |
| 4-Amino-N-benzoylbenzenesulfonamide |
| N-Benzoylsulfanilamide |
| Sulfabenzamide |
| N-(4-aminophenyl)sulfonylbenzamide |
| MFCD00044890 |
| Sulfabenzamide(SB) |
| Sultrin |
| EINECS 204-859-4 |
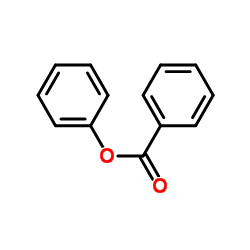 CAS#:93-99-2
CAS#:93-99-2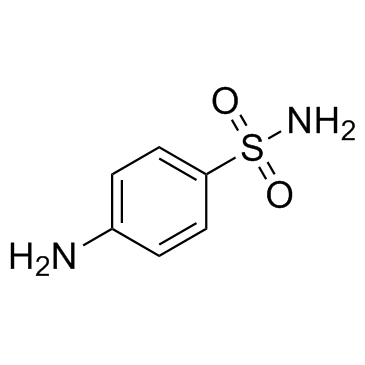 CAS#:63-74-1
CAS#:63-74-1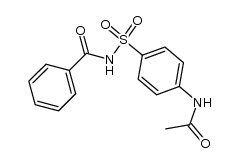 CAS#:5661-33-6
CAS#:5661-33-6 CAS#:98-88-4
CAS#:98-88-4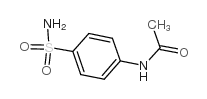 CAS#:121-61-9
CAS#:121-61-9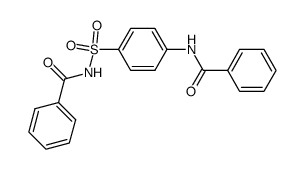 CAS#:724423-50-1
CAS#:724423-50-1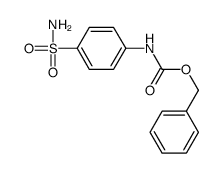 CAS#:55871-46-0
CAS#:55871-46-0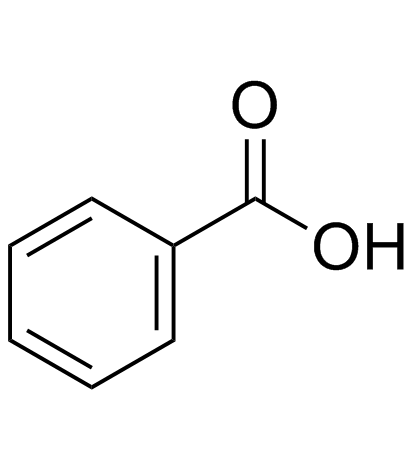 CAS#:65-85-0
CAS#:65-85-0 CAS#:623-11-0
CAS#:623-11-0
