CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WO8400000
-
CHEMICAL NAME :
-
Sulfanilamide
-
CAS REGISTRY NUMBER :
-
63-74-1
-
BEILSTEIN REFERENCE NO. :
-
0511852
-
LAST UPDATED :
-
199701
-
DATA ITEMS CITED :
-
30
-
MOLECULAR FORMULA :
-
C6-H8-N2-O2-S
-
MOLECULAR WEIGHT :
-
172.22
-
WISWESSER LINE NOTATION :
-
ZSWR DZ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3900 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2900 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
3130 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
180 gm/kg/90D-I
-
TOXIC EFFECTS :
-
Blood - changes in spleen Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
82200 mg/kg/39W-I
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - other changes
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
60 gm/kg/30D-I
-
TOXIC EFFECTS :
-
Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
135 mg/kg/9W-I
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
974 mg/kg
-
SEX/DURATION :
-
female 34-37 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - blood and lymphatic systems (including spleen and marrow)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
22 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
22 mg/kg
-
SEX/DURATION :
-
female 1-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
Cytogenetic analysis
MUTATION DATA
-
TYPE OF TEST :
-
DNA damage
-
TEST SYSTEM :
-
Rodent - rat Liver
-
DOSE/DURATION :
-
30 umol/L
-
REFERENCE :
-
SinJF# Personal Communication from J.F. Sina, Merck Institute for Therapeutic Research, West Point, PA 19486, Oct. 26, 1982 Volume(issue)/page/year: 26OCT1982 *** REVIEWS *** TOXICOLOGY REVIEW JMSHAO Journal of the Mount Sinai Hospital (New York). (New York, NY) V.1-36, 1934-69. For publisher information, see MSJMAZ. Volume(issue)/page/year: 10,343,1943 TOXICOLOGY REVIEW PCNAA8 Pediatric Clinics of North America. (W.B. Saunders Co., W. Washington Sq., Philadelphia, PA 19105) V.1- 1954- Volume(issue)/page/year: 8,413,1961 TOXICOLOGY REVIEW DPIRDU Dangerous Properties of Industrial Materials Report. (Van Nostrand Reinhold, 115 Fifth Ave., New York, NY 10003) V.1- 1981- Volume(issue)/page/year: 2(6),13,1982 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 84382 No. of Facilities: 149 (estimated) No. of Industries: 3 No. of Occupations: 9 No. of Employees: 3565 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 84382 No. of Facilities: 654 (estimated) No. of Industries: 6 No. of Occupations: 11 No. of Employees: 6529 (estimated) No. of Female Employees: 3131 (estimated)
|
 CAS#:6325-93-5
CAS#:6325-93-5 CAS#:352542-64-4
CAS#:352542-64-4 CAS#:121-61-9
CAS#:121-61-9 CAS#:98-64-6
CAS#:98-64-6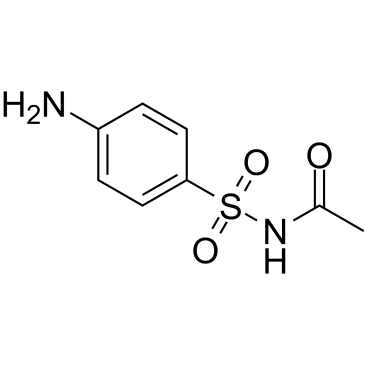 CAS#:144-80-9
CAS#:144-80-9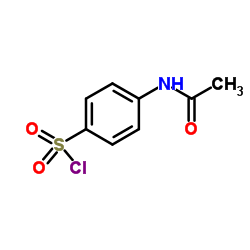 CAS#:121-60-8
CAS#:121-60-8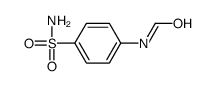 CAS#:829-72-1
CAS#:829-72-1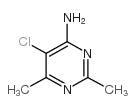 CAS#:2858-20-0
CAS#:2858-20-0![4-[5-(4-methylphenyl)-3-phenylpyrazol-1-yl]benzenesulfonamide structure](https://image.chemsrc.com/caspic/012/111607-61-5.png) CAS#:111607-61-5
CAS#:111607-61-5 CAS#:111456-11-2
CAS#:111456-11-2 CAS#:57-67-0
CAS#:57-67-0 CAS#:32933-40-7
CAS#:32933-40-7 CAS#:59-40-5
CAS#:59-40-5 CAS#:4392-54-5
CAS#:4392-54-5 CAS#:32909-92-5
CAS#:32909-92-5 CAS#:339-43-5
CAS#:339-43-5 CAS#:57-68-1
CAS#:57-68-1 CAS#:40724-47-8
CAS#:40724-47-8
