Flutamide
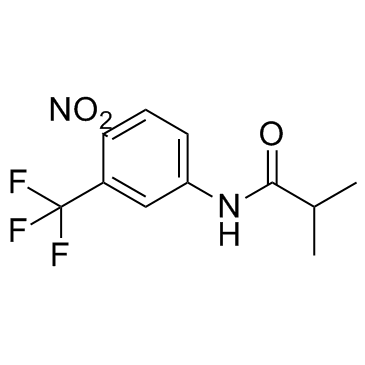
Flutamide structure
|
Common Name | Flutamide | ||
|---|---|---|---|---|
| CAS Number | 13311-84-7 | Molecular Weight | 276.212 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 400.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C11H11F3N2O3 | Melting Point | 112 °C | |
| MSDS | Chinese USA | Flash Point | 195.9±28.7 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of FlutamideFlutamide is an antiandrogen drug, with its active metablolite binding at androgen receptor with Ki values of 55 nM, and primarily used to treat prostate cancer.Target: androgen receptor in vitro: Flutamide (Eulexin) is an antiandrogen drug. Flutamide-OH, the active metabolite of flutamide, directly binds at rat anterior pituitary androgen receptor with Ki values of 55 nM [1]. lutamide does not affect the proliferation of an androgen-sensitive clone of the mouse mammary carcinoma Shionogi SC-l 15 cells in culture, shows only antiandrogenic effect, but not androgenic effect [2]. Flutamide provides treatment for prostate cancer when used along with leuprolide [3].in vivo: Flutamide causes a markedly reduction in rat ventral prostate weight from 319 mg to 245 mg. A combination of flutamide and LHRH agonist induces an additive effect with a decrease in prostate weight to 101 mg, and an marked drop in prostatic ODC activity [4]. |
| Name | flutamide |
|---|---|
| Synonym | More Synonyms |
| Description | Flutamide is an antiandrogen drug, with its active metablolite binding at androgen receptor with Ki values of 55 nM, and primarily used to treat prostate cancer.Target: androgen receptor in vitro: Flutamide (Eulexin) is an antiandrogen drug. Flutamide-OH, the active metabolite of flutamide, directly binds at rat anterior pituitary androgen receptor with Ki values of 55 nM [1]. lutamide does not affect the proliferation of an androgen-sensitive clone of the mouse mammary carcinoma Shionogi SC-l 15 cells in culture, shows only antiandrogenic effect, but not androgenic effect [2]. Flutamide provides treatment for prostate cancer when used along with leuprolide [3].in vivo: Flutamide causes a markedly reduction in rat ventral prostate weight from 319 mg to 245 mg. A combination of flutamide and LHRH agonist induces an additive effect with a decrease in prostate weight to 101 mg, and an marked drop in prostatic ODC activity [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 400.3±45.0 °C at 760 mmHg |
| Melting Point | 112 °C |
| Molecular Formula | C11H11F3N2O3 |
| Molecular Weight | 276.212 |
| Flash Point | 195.9±28.7 °C |
| Exact Mass | 276.072174 |
| PSA | 74.92000 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.521 |
| InChIKey | MKXKFYHWDHIYRV-UHFFFAOYSA-N |
| SMILES | CC(C)C(=O)Nc1ccc([N+](=O)[O-])c(C(F)(F)F)c1 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H361 |
| Precautionary Statements | P261-P280-P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R20/21/22;R63 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UG5700000 |
| HS Code | 2924299090 |
|
~79% 
Flutamide CAS#:13311-84-7 |
| Literature: Bandgar; Sawant Synthetic Communications, 2006 , vol. 36, # 7 p. 859 - 864 |
|
~% 
Flutamide CAS#:13311-84-7 |
| Literature: Synthetic Communications, , vol. 36, # 7 p. 859 - 864 |
|
~% 
Flutamide CAS#:13311-84-7 |
| Literature: Journal of Chemical Research, , vol. 38, # 4 p. 200 - 201 |
|
~% 
Flutamide CAS#:13311-84-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 10, p. 93 - 95 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Evolutionary history and functional characterization of androgen receptor genes in jawed vertebrates.
Endocrinology 150 , 5415-27, (2009) Vertebrates show diverse sexual characters in sexually attractive and reproductive organs, which are regulated by steroid hormones, particularly androgens. However, the evolutionary history of androge... |
|
|
Extending an in vitro panel for estrogenicity testing: the added value of bioassays for measuring antiandrogenic activities and effects on steroidogenesis.
Toxicol. Sci. 141(1) , 78-89, (2014) In the present study, a previously established integrated testing strategy (ITS) for in vitro estrogenicity testing was extended with additional in vitro assays in order to broaden its sensitivity to ... |
|
|
Inhibition of SGK1 enhances mAR-induced apoptosis in MCF-7 breast cancer cells.
Cancer Biol. Ther. 16(1) , 52-9, (2015) Functional membrane androgen receptors (mAR) have previously been described in MCF-7 breast cancer cells. Their stimulation by specific testosterone albumin conjugates (TAC) activate rapidly non-genom... |
| 2-Methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propanamide |
| 4'-Nitro-3'-(trifluoromethyl)isobutyranilide |
| MFCD00072009 |
| Propanamide, 2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]- |
| Eulexin |
| FXFFR BNW EMVY1&1 |
| Flutamidum |
| 2-Methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]propionamide |
| Drogenil |
| Niftholide |
| [14C]-Flutamide |
| Flutamide |
| NFBA |
| 4'-nitro-3'-trifluoromethylisobutyranilide |
| 2-Methyl-N-(4-nitro-3-[trifluoromethyl]phenyl)propanamide |
| Niftolide |
| niftolid |
| 2-Methyl-N-(4-nitro-3-[trifluoromethyl]phenyl)propanamide,Flutamide |
| Flutamin |
| EINECS 236-341-9 |
| Flutamida |
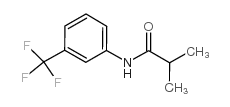
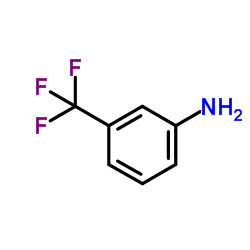

 CAS#:1579-89-1
CAS#:1579-89-1![N-[4-Amino-3-(trifluoromethyl)phenyl]-2-methylpropanamide structure](https://image.chemsrc.com/caspic/261/39235-51-3.png) CAS#:39235-51-3
CAS#:39235-51-3
