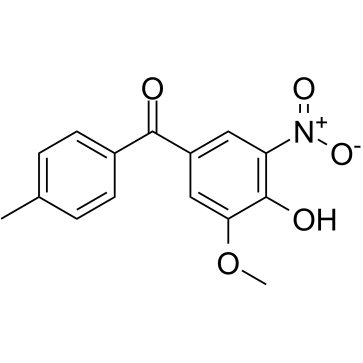
Tolcapone
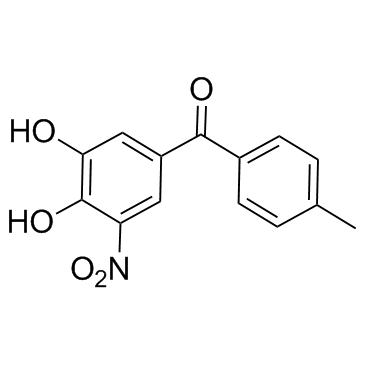
Tolcapone structure
|
Common Name | Tolcapone | ||
|---|---|---|---|---|
| CAS Number | 134308-13-7 | Molecular Weight | 273.241 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 485.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C14H11NO5 | Melting Point | 126-128ºC | |
| MSDS | Chinese USA | Flash Point | 205.7±17.2 °C | |
| Symbol |

GHS09 |
Signal Word | Warning | |
Use of TolcaponeTolcapone(Ro 40-7592) is an orally active selective, potent catechol-O-methyltransferase (COMT) inhibitor. IC50 value:Target: COMTTolcapone inhibits both central and peripheral COMT. Tolcapone caused a rapid and reversible inhibition of COMT activity in erythrocytes in parallel with a dose-dependent decrease in the formation of 3-OMD. Tolcapone increased the area under the concentration-time curve and elimination half-life of levodopa. Tolcapone crosses the blood-brain barrier, and has been used for L-DOPA adjunct therapy in the treatment of Parkinson's disease. |
| Name | (3,4-dihydroxy-5-nitrophenyl)-(4-methylphenyl)methanone |
|---|---|
| Synonym | More Synonyms |
| Description | Tolcapone(Ro 40-7592) is an orally active selective, potent catechol-O-methyltransferase (COMT) inhibitor. IC50 value:Target: COMTTolcapone inhibits both central and peripheral COMT. Tolcapone caused a rapid and reversible inhibition of COMT activity in erythrocytes in parallel with a dose-dependent decrease in the formation of 3-OMD. Tolcapone increased the area under the concentration-time curve and elimination half-life of levodopa. Tolcapone crosses the blood-brain barrier, and has been used for L-DOPA adjunct therapy in the treatment of Parkinson's disease. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 485.6±45.0 °C at 760 mmHg |
| Melting Point | 126-128ºC |
| Molecular Formula | C14H11NO5 |
| Molecular Weight | 273.241 |
| Flash Point | 205.7±17.2 °C |
| Exact Mass | 273.063721 |
| PSA | 103.35000 |
| LogP | 4.07 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.661 |
| InChIKey | MIQPIUSUKVNLNT-UHFFFAOYSA-N |
| SMILES | Cc1ccc(C(=O)c2cc(O)c(O)c([N+](=O)[O-])c2)cc1 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~92% 
Tolcapone CAS#:134308-13-7 |
| Literature: Synthetic Communications, , vol. 38, # 5 p. 810 - 815 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Real-time concurrent monitoring of apoptosis, cytosolic calcium, and mitochondria permeability transition for hypermulticolor high-content screening of drug-induced mitochondrial dysfunction-mediated hepatotoxicity.
Toxicol. Lett. 214(2) , 175-81, (2012) A quantitative high-content screening (HCS) was suggested for the real-time monitoring of drug-induced mitochondrial dysfunction-mediated hepatotoxicity. This HCS is very advantageous in that it allow... |
|
|
Telescoping phenomenon in pathological gambling: association with gender and comorbidities.
J. Nerv. Ment. Dis. 200(11) , 996-8, (2012) The course of pathological gambling (PG) in women has been described as having a later age of initiation but a shorter time to problematic gambling ("telescoped"). This study examined evidence for tel... |
|
|
Capture compound mass spectrometry sheds light on the molecular mechanisms of liver toxicity of two Parkinson drugs.
Toxicol. Sci. 113(1) , 243-53, (2010) Capture compound mass spectrometry (CCMS) is a novel technology that helps understand the molecular mechanism of the mode of action of small molecules. The Capture Compounds are trifunctional probes: ... |
| UNII-CIF6334OLY |
| 1-(3,4-dihydroxy-5-nitrophenyl)-1-(4-methylphenyl)methanone |
| tolcapone |
| Methanone, (3,4-dihydroxy-5-nitrophenyl)(4-methylphenyl)- |
| tolcapone [INN_en] |
| 3,4-Dihydroxy-4'-methyl-5-nitrobenzophenone |
| 4'-methyl-3,4-dihydroxy-5-nitro-benzophenone |
| [14C]-Tolcapone |
| Tasmar (TN) |
| MFCD00866569 |
| Methanone,(3,4-dihydroxy-5-nitrophenyl)(4-methylphenyl) |
| Tasmar |
| (3,4-Dihydroxy-5-nitrophenyl)(4-methylphenyl)methanone |
| Ro 40-7592 |