134308-13-7
| Name | (3,4-dihydroxy-5-nitrophenyl)-(4-methylphenyl)methanone |
|---|---|
| Synonyms |
UNII-CIF6334OLY
1-(3,4-dihydroxy-5-nitrophenyl)-1-(4-methylphenyl)methanone tolcapone tolcapone [INN_en] 3,4-Dihydroxy-4'-methyl-5-nitrobenzophenone 4'-methyl-3,4-dihydroxy-5-nitro-benzophenone [14C]-Tolcapone Tasmar (TN) MFCD00866569 Methanone,(3,4-dihydroxy-5-nitrophenyl)(4-methylphenyl) Tasmar (3,4-Dihydroxy-5-nitrophenyl)(4-methylphenyl)methanone Ro 40-7592 |
| Description | Tolcapone(Ro 40-7592) is an orally active selective, potent catechol-O-methyltransferase (COMT) inhibitor. IC50 value:Target: COMTTolcapone inhibits both central and peripheral COMT. Tolcapone caused a rapid and reversible inhibition of COMT activity in erythrocytes in parallel with a dose-dependent decrease in the formation of 3-OMD. Tolcapone increased the area under the concentration-time curve and elimination half-life of levodopa. Tolcapone crosses the blood-brain barrier, and has been used for L-DOPA adjunct therapy in the treatment of Parkinson's disease. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 485.6±45.0 °C at 760 mmHg |
| Melting Point | 126-128ºC |
| Molecular Formula | C14H11NO5 |
| Molecular Weight | 273.241 |
| Flash Point | 205.7±17.2 °C |
| Exact Mass | 273.063721 |
| PSA | 103.35000 |
| LogP | 4.07 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.661 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H400 |
| Precautionary Statements | P273 |
| Hazard Codes | N |
| Risk Phrases | 50 |
| Safety Phrases | 61 |
| RIDADR | UN 3077 9 / PGIII |
| RTECS | PC4952500 |
|
~92% 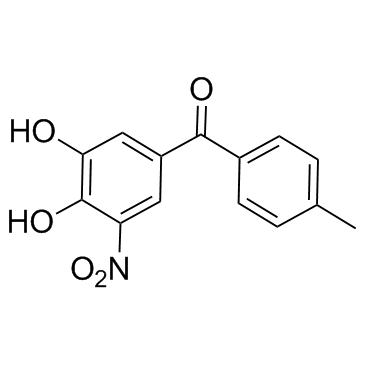
134308-13-7 |
| Literature: Synthetic Communications, , vol. 38, # 5 p. 810 - 815 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |