Aloin
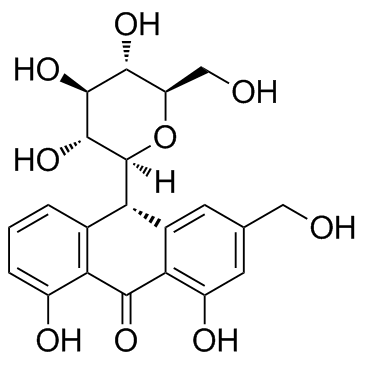
Aloin structure
|
Common Name | Aloin | ||
|---|---|---|---|---|
| CAS Number | 1415-73-2 | Molecular Weight | 418.394 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 752.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C21H22O9 | Melting Point | 148-149ºC | |
| MSDS | Chinese USA | Flash Point | 268.0±26.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AloinAloin(Aloin-A; Barbaloin-A) is a natural antitumor anthraquinone glycoside with iron chelating and non-atherogenic activities.IC50 value:Target:in vitro: Aloin significantly inhibited HUVECs proliferation, migration and tube formation in vitro. suppressed activation of VEGF receptor (VEGFR) 2 and STAT3 phosphorylation in endothelial cells. In addition, the constitutively activated STAT3 protein, and the expression of STAT3-regulated antiapoptotic (Bcl-xL), proliferative (c-Myc), and angiogenic (VEGF) proteins were also down-regulated in response to AL in human SW620 cancer cells [1]. aloin exerted inhibition of cell proliferation, adhesion and invasion abilities of B16-F10 melanoma cells under non-cytotoxic concentrations. Furthermore, aloin induced melanoma cell differentiation through the enhancement of melanogenesis and transglutaminase activity [2].in vivo: Aloin substantially reduced tumor volumes and weight in vivo mouse xenografts, without obviously toxicity [1]. Aloin (10, 30 mg/kg bw) or vehicle was given by gavage to mice after each alcohol administration. Alcohol elevated the serum transaminases alanine aminotransferase, aspartate aminotransferase, total cholesterol and triglyceride levels which were significantly attenuated by the co-administration of aloin (p < 0.05) [3]. |
| Name | aloin A |
|---|---|
| Synonym | More Synonyms |
| Description | Aloin(Aloin-A; Barbaloin-A) is a natural antitumor anthraquinone glycoside with iron chelating and non-atherogenic activities.IC50 value:Target:in vitro: Aloin significantly inhibited HUVECs proliferation, migration and tube formation in vitro. suppressed activation of VEGF receptor (VEGFR) 2 and STAT3 phosphorylation in endothelial cells. In addition, the constitutively activated STAT3 protein, and the expression of STAT3-regulated antiapoptotic (Bcl-xL), proliferative (c-Myc), and angiogenic (VEGF) proteins were also down-regulated in response to AL in human SW620 cancer cells [1]. aloin exerted inhibition of cell proliferation, adhesion and invasion abilities of B16-F10 melanoma cells under non-cytotoxic concentrations. Furthermore, aloin induced melanoma cell differentiation through the enhancement of melanogenesis and transglutaminase activity [2].in vivo: Aloin substantially reduced tumor volumes and weight in vivo mouse xenografts, without obviously toxicity [1]. Aloin (10, 30 mg/kg bw) or vehicle was given by gavage to mice after each alcohol administration. Alcohol elevated the serum transaminases alanine aminotransferase, aspartate aminotransferase, total cholesterol and triglyceride levels which were significantly attenuated by the co-administration of aloin (p < 0.05) [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 752.6±60.0 °C at 760 mmHg |
| Melting Point | 148-149ºC |
| Molecular Formula | C21H22O9 |
| Molecular Weight | 418.394 |
| Flash Point | 268.0±26.4 °C |
| Exact Mass | 418.126373 |
| PSA | 167.91000 |
| LogP | 1.86 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.741 |
| Stability | Stable, but light sensitive. Incompatible with bases, strong oxidizing agents. Combustible. |
| Water Solubility | pyridine: 50 mg/mL, clear, dark red |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | LZ6520000 |
|
~% 
Aloin CAS#:1415-73-2 |
| Literature: Pharmaceutica Acta Helvetiae, , vol. 27, p. 17,23 |
|
~% 
Aloin CAS#:1415-73-2 |
| Literature: Pharmaceutica Acta Helvetiae, , vol. 27, p. 17,23 |
|
The content of secondary phenol metabolites in pruned leaves of Aloe arborescens, a comparison between two methods: leaf exudates and leaf water extract.
J. Nat. Med. 62(4) , 430-5, (2008) Aloe arborescens plants, originating from the deserts of South Africa, are grown in the Introduction Garden at Sede Boker in the Negev Desert of Israel. In previous studies, we developed agro-technica... |
|
|
Determination of aloenin, barbaloin and isobarbaloin in aloe species by micellar electrokinetic chromatography.
J. Chromatogr. B. Biomed. Sci. Appl. 752(1) , 91-7, (2001) Aloenin, barbaloin and isobarbaloin in JP Aloe, Aloe barbadensis (Aloe vera) and Aloe arborescens Miller var. natalensis Berger (Aloe arborescens Miller) were determined by micellar electrokinetic chr... |
|
|
Liquid chromatographic determination of barbaloin (aloin) in foods.
J. Assoc. Off. Anal. Chem. 68(3) , 493-4, (1985) A simple and rapid liquid chromatographic method is described for the determination of barbaloin (aloin, 10-D-glucopyranosyl-1,8-dihydroxy-3-(hydroxymethyl)-9(10H)-anthraceno ne) in foods. Barbaloin i... |
| (1S)-1,5-Anhydro-1-[(9R)-4,5-dihydroxy-2-(hydroxymethyl)-10-oxo-9,10-dihydroanthracen-9-yl]-D-glucitol |
| (10R)-10-β-D-Glucopyranosyl-1,8-dihydroxy-3-(hydroxymethyl)-9(10H)-anthracenone |
| EINECS 215-808-0 |
| barbaloin isomers |
| BAR-5'-monophosphate |
| N6-Benzyladenosin-5'-phosphat |
| (10R)-1,8-Dihydroxy-3-(hydroxymethyl)-10-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-9(10H)-anthracenone |
| Aloin |
| 10-β-D-Glucopyranosyl-1,8-dihydroxy-3-(hydroxymethyl)-9(10H)-anthracenone |
| Benzyl-amp |
| isobarbaloin |
| Benzyl-adenosine monophosphate |
| 9(10H)-Anthracenone, 10-β-D-glucopyranosyl-1,8-dihydroxy-3-(hydroxymethyl)-, (R)- |
| MFCD00151160 |
| 10-(1',5'-Anhydroglucosyl)-aloe-emodin-9-anthrone |
| 6-N-benzyladenosine-5'-O-monophosphate |
| N6-Benzyladenosin-5'-monophosphat |
| N6-Benzyladenosine 5'-Phosphat |
| D-Glucitol, 1,5-anhydro-1-C-[(9R)-9,10-dihydro-4,5-dihydroxy-2-(hydroxymethyl)-10-oxo-9-anthracenyl]-, (1S)- |
| 1,8-Dihydroxy-10-(β-D-glucopyranosyl)-3-(hydroxymethyl)-9(10H)-anthracenone |
| (1S)-1,5-Anhydro-1-[(9R)-4,5-dihydroxy-2-(hydroxymethyl)-10-oxo-9,10-dihydro-9-anthracenyl]-D-glucitol |
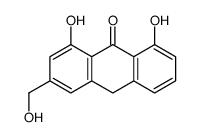
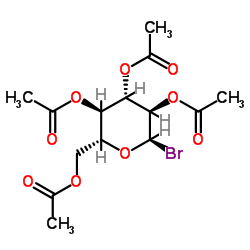
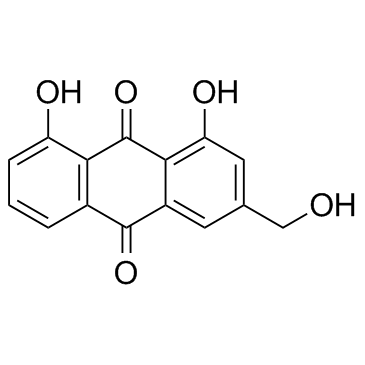 CAS#:481-72-1
CAS#:481-72-1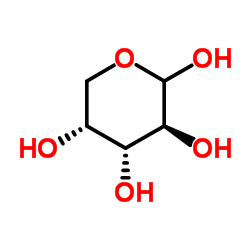 CAS#:10323-20-3
CAS#:10323-20-3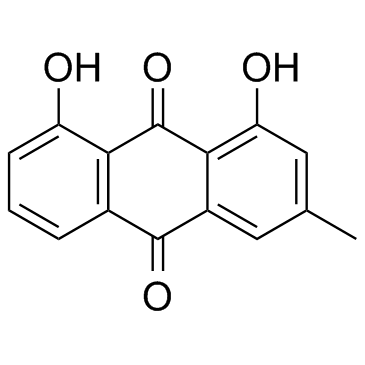 CAS#:481-74-3
CAS#:481-74-3 CAS#:65615-58-9
CAS#:65615-58-9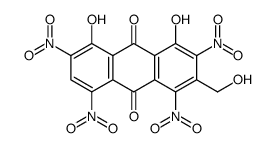 CAS#:567-95-3
CAS#:567-95-3
