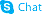Dexmedetomidine hydrochloride

Dexmedetomidine hydrochloride structure
|
Common Name | Dexmedetomidine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 145108-58-3 | Molecular Weight | 236.740 | |
| Density | N/A | Boiling Point | 381.9ºC at 760 mmHg | |
| Molecular Formula | C13H17ClN2 | Melting Point | 153 - 158ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Dexmedetomidine hydrochlorideDexmedetomidine Hydrochloride is an agonist of adrenergic alpha-2 receptor, which is used in veterinary medicine for its analgesic and sedative properties.Target: Adrenergic alpha-2 ReceptorDexmedetomidine, acting at alpha(2A) adrenoceptors, must be present during the encoding process to decrease discrete cue fear memory; however, its ability to suppress contextual memory is likely the result of blocking the consolidation process [1]. Dexmedetomidine had no analgesic effects in alpha(2A)-adrenoceptor KO mice [2]. Dexmedetomidine was effective in blocking these sympathomimetic actions of cocaine even in all 7 subjects who were homozygous for the Del322-325 polymorphism in the alpha2C AR, a loss-of-function mutation that is highly enriched in blacks [3]. |
| Name | dexmedetomidine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Dexmedetomidine Hydrochloride is an agonist of adrenergic alpha-2 receptor, which is used in veterinary medicine for its analgesic and sedative properties.Target: Adrenergic alpha-2 ReceptorDexmedetomidine, acting at alpha(2A) adrenoceptors, must be present during the encoding process to decrease discrete cue fear memory; however, its ability to suppress contextual memory is likely the result of blocking the consolidation process [1]. Dexmedetomidine had no analgesic effects in alpha(2A)-adrenoceptor KO mice [2]. Dexmedetomidine was effective in blocking these sympathomimetic actions of cocaine even in all 7 subjects who were homozygous for the Del322-325 polymorphism in the alpha2C AR, a loss-of-function mutation that is highly enriched in blacks [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 381.9ºC at 760 mmHg |
|---|---|
| Melting Point | 153 - 158ºC |
| Molecular Formula | C13H17ClN2 |
| Molecular Weight | 236.740 |
| Exact Mass | 236.108032 |
| PSA | 28.68000 |
| LogP | 3.98030 |
| Vapour Pressure | 1.08E-05mmHg at 25°C |
| Storage condition | Desiccate at RT |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P301 + P312 + P330-P305 + P351 + P338 |
| Hazard Codes | Xn |
| Risk Phrases | 22-36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | NONH for all modes of transport |
| RTECS | NI5156750 |
|
Effect of corneal contact lens wear on healing time and comfort post LGK for treatment of SCCEDs in boxers.
Vet. Ophthalmol. 18 , 364-70, (2015) To determine whether dogs with spontaneous chronic corneal epithelial defects (SCCEDs) would heal faster and with an improved comfort score following linear grid keratotomy (LGK) combined with corneal... |
|
|
Repeated exposure to MDMA triggers long-term plasticity of noradrenergic and serotonergic neurons.
Mol. Psychiatry 19(7) , 823-33, (2014) 3,4-Methylenedioxymethamphetamine (MDMA or 'ecstasy') is a psychostimulant drug, widely used recreationally among young people in Europe and North America. Although its neurotoxicity has been extensiv... |
|
|
Optimized on-line enantioselective capillary electrophoretic method for kinetic and inhibition studies of drug metabolism mediated by cytochrome P450 enzymes.
Electrophoresis 36 , 1349-57, (2015) Pharmacokinetic and pharmacodynamic properties of a chiral drug can significantly differ between application of the racemate and single enantiomers. During drug development, the characteristics of can... |
| 4-[(a-Methyl)-2,3-dimethylbenzyl]imidazole Monohydrochloride |
| 4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole hydrochloride (1:1) |
| Dexdor |
| 1H-imidazole, 4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-, monohydrochloride |
| 5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole,hydrochloride |
| MFCD07772269 |
| 1H-Imidazole, 4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-, hydrochloride (1:1) |
| UNII:BH210P244U |
| UNII-1018WH7F9I |
| 4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole Monohydrochloride |
| 5-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole hydrochloride |
| Precedex |
| (S)-4-(1-(2,3-Dimethylphenyl)ethyl)-1H-imidazole Monohydrochloride |
| 1H-Imidazole, 4-[1-(2,3-dimethylphenyl)ethyl]-, hydrochloride (1:1) |
| Dexmedetomidine HCL |
| 4-[(1S)-1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole hydrochloride (1:1) |
| 4-((S)-a,2,3-Trimethylbenzyl)imidazole Monohydrochloride |
| (±)-4-(a,2,3-Trimethylbenzyl)imidazole Monohydrochloride |
| Dexmedetomidine Hydrochloride |
| 4-[(1S)-1-(2,3-Dimethylphenyl)ethyl]-1H-imidazolhydrochlorid |
| 4-[(1S)-1-(2,3-diméthylphényl)éthyl]-1H-imidazole chlorhydrate |
| Dexmedetomidine HCl |
| 4-(S)-[1-(2,3-DIMETHYLPHENYL)ETHYL]-1 H-IMIDAZOLE MONOHY DROCHLORIDE (DEXMEDETOMIDINE HYDROCHLORIDE) |
| Dexmedetomidine (hydrochloride) |