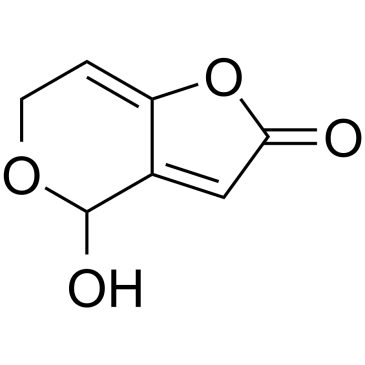
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
LV2625000
-
CHEMICAL NAME :
-
4H-Furo(3,2-c)pyran-2(6H)-one, 4-hydroxy-
-
CAS REGISTRY NUMBER :
-
149-29-1
-
BEILSTEIN REFERENCE NO. :
-
0149675
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
59
-
MOLECULAR FORMULA :
-
C7-H6-O4
-
MOLECULAR WEIGHT :
-
154.13
-
WISWESSER LINE NOTATION :
-
T56 BOV GO IU&TJ FQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
27790 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Gastrointestinal - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4590 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Gastrointestinal - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
11 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Lungs, Thorax, or Respiration - dyspnea Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
8570 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Lungs, Thorax, or Respiration - dyspnea Gastrointestinal - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
17 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - acute pulmonary edema Liver - fatty liver degeneration Kidney, Ureter, Bladder - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - excitement Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - respiratory stimulation
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intracerebral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
570 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - cyanosis
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
8 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - food intake (animal) Gastrointestinal - hypermotility, diarrhea
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - pleural effusion
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
31500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
23 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Bird - chicken
-
DOSE/DURATION :
-
170 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
392 mg/kg/14D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - alteration in gastric secretion Gastrointestinal - gastritis Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
84 mg/kg/4W-C
-
TOXIC EFFECTS :
-
Brain and Coverings - recordings from specific areas of CNS Kidney, Ureter, Bladder - other changes in urine composition Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
60 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases Biochemical - Metabolism (Intermediary) - lipids including transport
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
232 mg/kg/58W-I
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
135 mg/kg
-
SEX/DURATION :
-
male 5 week(s) pre-mating female 5 week(s) pre-mating - 20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
675 mg/kg
-
SEX/DURATION :
-
male 5 week(s) pre-mating female 5 week(s) pre-mating - 20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
135 mg/kg
-
SEX/DURATION :
-
multigenerations
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
24 mg/kg
-
SEX/DURATION :
-
female 14-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
18 mg/kg
-
SEX/DURATION :
-
female 6-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
24 mg/kg
-
SEX/DURATION :
-
female 6-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
Sex chromosome loss and nondisjunction
-
TYPE OF TEST :
-
Cytogenetic analysis
MUTATION DATA
-
TYPE OF TEST :
-
Micronucleus test
-
TEST SYSTEM :
-
Mammal - domestic Cells - not otherwise specified
-
DOSE/DURATION :
-
500 nmol/L
-
REFERENCE :
-
TOXID9 Toxicologist. (Soc. of Toxicology, Inc., 475 Wolf Ledge Parkway, Akron, OH 44311) V.1- 1981- Volume(issue)/page/year: 13,219,1993 *** REVIEWS *** IARC Cancer Review:Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 40,83,1986 IARC Cancer Review:Human No Adequate Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 40,83,1986 IARC Cancer Review:Group 3 IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,56,1987 TOXICOLOGY REVIEW LLOYA2 Lloydia. (Cincinnati, OH) V.1-41, 1938-78. For publisher information, see JNPRDF. Volume(issue)/page/year: 38,21,1975 TOXICOLOGY REVIEW MUREAV Mutation Research. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1964- Volume(issue)/page/year: 26,225,1974 TOXICOLOGY REVIEW JMFTAT Journal of Milk and Food Technology. (Ames, IA) V.10-39, 1947-76. Volume(issue)/page/year: 38,695,1975 TOXICOLOGY REVIEW 22HAAD "Microbial Toxins, 1970-72," Ajil, S.J., et al., eds., New York, Academic Press, Inc., 1970-72 Volume(issue)/page/year: 6,409,1971 TOXICOLOGY REVIEW ARMIAZ Annual Review of Microbiology. (Annual Reviews, Inc., POB 10139, Palo Alto, CA 94303) V.1- 1947- Volume(issue)/page/year: 26,279,1972
|