| Description |
Bendamustine (SDX-105 free base), a purine analogue, is a DNA cross-linking agent. Bendamustine activates DNA-damage stress response and apoptosis. Bendamustine has potent alkylating, anticancer and antimetabolite properties[1].
|
| Related Catalog |
|
| Target |
DNA Alkylator/Crosslinker[1]
|
| In Vitro |
Bendamustine is a DNA cross-linking agent that causes DNA breaks, with alkylating and antimetabolite properties. Bendamustine uniquely regulates apoptosis pathways and DNA repair pathways in non-Hodgkin's lymphoma cells. Bendamustine (50 μM) induces p21 (Cip1/Waf1) and NOXA genes, and increases the expression of p53 in SU-DHL-1 cells. Bendamustine (25 μM) blocks mitotic checkpoints and cuases mitotic catastrophe[1]. Bendamustine reduces the viability of multiple myeloma (MM) cell lines, such as RPMI-8226 and 8226-LR5 cells, with IC25s of 101.8 μM and 585.5 μM after 24 h incubation, and 51.7 and 374.3 μM after 48 h incubation, respectively. Bendamustine induces a specific caspase-dependent MM cell death and inhibits the spindle-assembly checkpoint[2].
|
| In Vivo |
Bendamustine (25 mg/kg, i.v.) shows potent inhibition on the growth of tumor cells by 91%, 99% and 95% for DoHH-2, Granta 519 and RAMOS models, respectively. Moreover, the antitumor effect of Bendamustine is enhanced by rituximab in DoHH-2 and RAMOS models, but not in Granta 519 model[3].
|
| Cell Assay |
Cytotoxicity of both Bendamustine and melphalan on multiple myeloma (MM) cells is calculated as inhibition of cell viability by measuring the percentage of cell survival by MTS assay. Briefly, cells (1 × 104/well) are seeded in 96-well plates with increasing concentrations of the drug and analyzed after 24, 48, 72 and 96 h of incubation. To this end, 1 μg/mL of MTS solution is added to each well and, after 1 h at 37 °C, the dark blue formazan crystals are dissolved by isopropanol 1 N and HCl (24:1, vol/vol). Finally, the absorbance is measured at 490 nm in a 96-well plate reader. Cell survival is estimated as the percentage of the absorbance of untreated controls and each test is performed in triplicate. The inhibitory concentrations 50 (IC50) and 25 (IC25) of each drug, being the amount able to reduce cell growth to 50% and 25%, respectively, of that of untreated control cells, are calculated, and the tests are performed in parallel using equitoxic concentrations of Bendamustine and melphalan. The relative resistance index (RRI) is expressed as the ratio of the IC50 of 8226-LR5 to the IC50 of RPMI-8226 cells[2].
|
| Animal Admin |
Mice[3] C.B.-17 scid mice (DoHH-2, Granta 519) or C.B.-17 scid-bg mice (SuDHL-4, RAMOS) are inoculated with 1 × 106 (DoHH-2, RAMOS), 3 × 106 (SuDHL-4) or 5 × 106 (Granta 519) cells s.c. in the right flank. For flank xenografts, inoculation volume is 0.2 mL consisting of a 50:50 mixture of cells in growth medium and Matrigel. Tumour volume is estimated by two to three weekly measurements of the length and width of the tumour by electronic calipers and applying the following equation: V=L×W2/2. Tumours are allowed to reach approximately 250 mm3, and mice are size-matched (day 0) into treatment and control groups. For systemic Granta 519 tumour models, 2 × 106 cells are injected via the tail vein in 0.1 mL volume of cell medium on day 0, and treatment is initiated on day 14. All animals are ear-tagged and monitored individually throughout the experiment. Navitoclax is administered by oral gavage once daily in a mixture of Phosal 50PG : PEG400 : ethanol. Bendamustine and rituximab are administered i.v. at 25 mg/kg and 10 mg/kg, respectively, on day 1. Navitoclax is administered approximately 2 h before Bendamustine and rituximab. All trials are comprised of 10 mice per group. Mice are humanely killed when tumours reach a size >2000 mm3 or when any signs of distress are monitored. Signs of distress include loss of ambulation, laboured breathing or weight loss > 20% mean body weight per cage[3].
|
| References |
[1]. Leoni LM, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008 Jan 1;14(1):309-17. [2]. Cives M, et al. Bendamustine overcomes resistance to melphalan in myeloma cell lines by inducing cell death through mitotic catastrophe. Cell Signal. 2013 May;25(5):1108-17. [3]. Ackler S, et al. Navitoclax (ABT-263) and bendamustine ± rituximab induce enhanced killing of non-Hodgkin's lymphoma tumours in vivo. Br J Pharmacol. 2012 Oct;167(4):881-91.
|

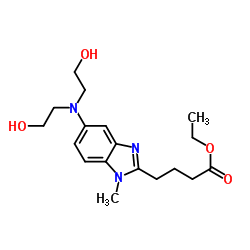 CAS#:3543-74-6
CAS#:3543-74-6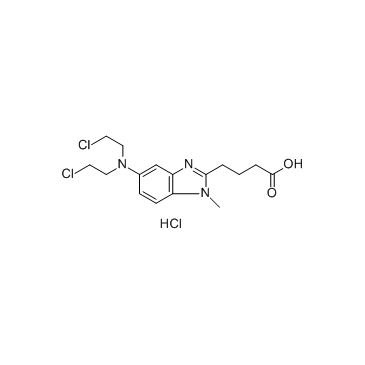 CAS#:3543-75-7
CAS#:3543-75-7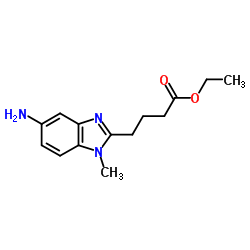 CAS#:3543-73-5
CAS#:3543-73-5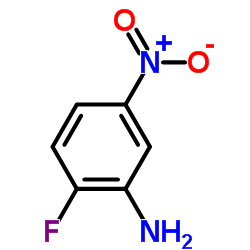 CAS#:369-36-8
CAS#:369-36-8 CAS#:108-55-4
CAS#:108-55-4 CAS#:31349-48-1
CAS#:31349-48-1 CAS#:3543-72-4
CAS#:3543-72-4![Methyl 4-{5-[bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2- yl}butanoate structure](https://image.chemsrc.com/caspic/156/109882-25-9.png) CAS#:109882-25-9
CAS#:109882-25-9