RUBOXISTAURIN MESYLATE
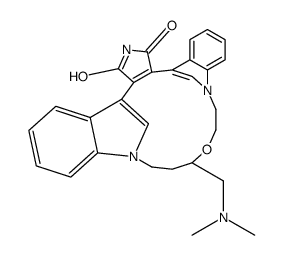
RUBOXISTAURIN MESYLATE structure
|
Common Name | RUBOXISTAURIN MESYLATE | ||
|---|---|---|---|---|
| CAS Number | 169939-94-0 | Molecular Weight | 468.54700 | |
| Density | 1.34g/cm3 | Boiling Point | 744.4ºC at 760mmHg | |
| Molecular Formula | C28H28N4O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 404ºC | |
Use of RUBOXISTAURIN MESYLATERuboxistaurin (LY333531) is an orally active, selective PKC beta inhibitor (Ki=2 nM). Ruboxistaurin exhibits ATP dependent competitive inhibition of PKC beta I with an IC50 of 4.7 nM. Ruboxistaurin inhibits PKC beta II with an IC50 of 5.9 nM[1][2]. |
| Name | Ruboxistaurin |
|---|---|
| Synonym | More Synonyms |
| Description | Ruboxistaurin (LY333531) is an orally active, selective PKC beta inhibitor (Ki=2 nM). Ruboxistaurin exhibits ATP dependent competitive inhibition of PKC beta I with an IC50 of 4.7 nM. Ruboxistaurin inhibits PKC beta II with an IC50 of 5.9 nM[1][2]. |
|---|---|
| Related Catalog | |
| Target |
PKC-βI:4.7 nM (IC50) PKC-βII:5.9 nM (IC50) PKCη:52 nM (IC50) PKCδ:250 nM (IC50) PKCγ:300 nM (IC50) PKCα:360 nM (IC50) PKCε:600 nM (IC50) |
| In Vitro | Ruboxistaurin is a selective and ATP-competitive PKCβ inhibitor, with IC50s of 4.7 and 5.9 nM for PKCβI and PKCβII, shows less potent inhibition on PKCη (IC50, 52 nM), PKCα (IC50, 360 nM), PKCγ (IC50, 300 nM), PKCδ (IC50, 250 nM), and has no effect on PKCζ (IC50, >100 μM)[1]. Ruboxistaurin (10 and 400 nM) dramatically inhibits glucose-induced monocyte adherence to levels that are not different from baseline adherence of monocytes to endothelial cells under NG conditions. Ruboxistaurin (10 and 400 nM) dose not alter the endothelial expression of adhesion molecules or modify endothelial cell growth[2]. Ruboxistaurin (LY333531; 10 nM) reduces high-glucose (HG)-induced human renal glomerular endothelial cells (HRGECs) viability, and inhibits the increases in swiprosin-1 in HRGECs incubated with HG[3]. |
| In Vivo | Ruboxistaurin (1 mg/kg; 8 weeks) markedly reduces GEC apoptosis as well as swiprosin-1 upregulation, and ameliorates renal glomerular injury in the diabetic mice. Ruboxistaurin also potently attenuates the expression of PARP, cleaved-caspase9, cleaved-caspase3, and the Bax/Bcl-2 ratio, in diabetic mice[3]. Ruboxistaurin (0.1, 1.0, or 10.0 mg/kg; p.o.) dramatically reduces the number of leukocytes trapped in the retinal microcirculation of diabetic rats[4]. Animal Model: Rats[4] Dosage: 0.1, 1.0, or 10.0 mg/kg Administration: P.o. Result: Dramatically reduced the number of leukocytes trapped in the retinal microcirculation of diabetic rats. |
| References |
[2]. Ruboxistaurin: LY 333531. Drugs R D. 2007;8(3):193-199. |
| Density | 1.34g/cm3 |
|---|---|
| Boiling Point | 744.4ºC at 760mmHg |
| Molecular Formula | C28H28N4O3 |
| Molecular Weight | 468.54700 |
| Flash Point | 404ºC |
| Exact Mass | 468.21600 |
| PSA | 71.99000 |
| LogP | 3.78960 |
| Vapour Pressure | 4.93E-22mmHg at 25°C |
| Index of Refraction | 1.695 |
| InChIKey | ZCBUQCWBWNUWSU-SFHVURJKSA-N |
| SMILES | CN(C)CC1CCn2cc(c3ccccc32)C2=C(C(=O)NC2=O)c2cn(c3ccccc23)CCO1 |
| 13-((Dimethylamino)methyl)-10,11,14,15-tetrahydro-4,9:16,21-dimetheno-1H,13H-dibenzo(e,k)pyrrolo(3,4-h)(1,4,13)oxadiazacyclohexadecene-1,3(2H)-dione |
| 1uu3 |