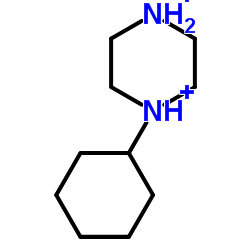
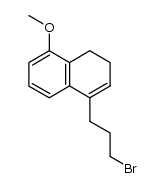
PB 28 dihydrochloride
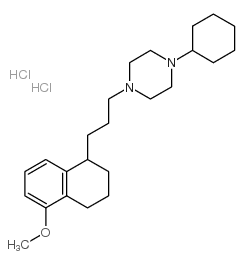
PB 28 dihydrochloride structure
|
Common Name | PB 28 dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 172906-90-0 | Molecular Weight | 443.49300 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C24H38N2O | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of PB 28 dihydrochloridePB28 is a cyclohexylpiperazine derivative and a high affinity and selective sigma 2 (σ2) receptor agonist with a Ki of 0.68 nM. PB28 is also a σ1 antagonist with a Ki of 0.38 nM. PB28 is less affinity for other receptors. PB28 inhibits electrically evoked twitch in guinea pig bladder and ileum with EC50 values of 2.62 μM and 3.96 μM, respectively. PB28 can modulate SARS-CoV-2-human protein-protein interaction. PB28 induces caspase-independent apoptosis and has antitumor activity[1][2][3][4][5]. |
| Name | PB28 dihydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | PB28 is a cyclohexylpiperazine derivative and a high affinity and selective sigma 2 (σ2) receptor agonist with a Ki of 0.68 nM. PB28 is also a σ1 antagonist with a Ki of 0.38 nM. PB28 is less affinity for other receptors. PB28 inhibits electrically evoked twitch in guinea pig bladder and ileum with EC50 values of 2.62 μM and 3.96 μM, respectively. PB28 can modulate SARS-CoV-2-human protein-protein interaction. PB28 induces caspase-independent apoptosis and has antitumor activity[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 0.68 nM (σ2 receptor); 0.38 nM (σ1 receptor)[4] |
| In Vitro | PB28 (15-25 nM; 24-48 hours; MCF7 and MCF7 ADR cells) treatment shows an accumulation in the G0-G1 phase for MCF7 and MCF7 ADR cells that are time and concentration independent[1]. PB28 has a higher σ2 receptor affinity expressed as Ki (0.28 nM and 0.17 nM in MCF7 and MCF7 ADR cells, respectively) than σ1 receptor affinity (13.0 nMand 10.0 nM, respectively)[1]. PB28 inhibits cell growth of MCF7 and MCF7 ADR cells with IC50s of 25 nM and 15 nM, respectively after 2-day treatment[1]. PB28 induces apoptosis through a caspase-independent pathway[1]. PB28 also reduces P-gp expression in a concentration- and time-dependent manner (approximately 60% in MCF7 and 90% in MCF7 ADR)[1]. PB28 displays antiproliferative and cytotoxic effects in both C6 rat glioma and SK-N-SH human neuroblastoma cell lines[1]. Cell Cycle Analysis[1] Cell Line: MCF7 and MCF7 ADR cells Concentration: 25 nM and 15 nM Incubation Time: 24 hours, 48 hours Result: Showed an accumulation in the G0-G1 phase for MCF7 and MCF7 ADR cells that were time and concentration independent. |
| In Vivo | PB28 (10.7 mg/mL; intraperitoneal injection; daily; for two weeks; C57BL/6 female mice) treatment inhibits tumor growth in Panc02 tumor burden mice. PB28 also conferres a survival advantage for mice[2]. Animal Model: C57BL/6 female mice (10 weeks old) injected with Panc02 cells[2] Dosage: 10.7 mg/mL Administration: Intraperitoneal injection; daily; for two weeks Result: Inhibited tumor growth in Panc02 tumor burden mice. |
| References |
| Molecular Formula | C24H38N2O |
|---|---|
| Molecular Weight | 443.49300 |
| Exact Mass | 442.25200 |
| PSA | 15.71000 |
| LogP | 6.32540 |
| InChIKey | PHRCDWVPTULQMT-UHFFFAOYSA-N |
| SMILES | COc1cccc2c1CCCC2CCCN1CCN(C2CCCCC2)CC1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
|
~70% 
PB 28 dihydroch... CAS#:172906-90-0 |
| Literature: Berardi; Colabufo; Giudice; Perrone; Tortorella; Govoni; Lucchi Journal of Medicinal Chemistry, 1996 , vol. 39, # 1 p. 176 - 182 |
|
~% 
PB 28 dihydroch... CAS#:172906-90-0 |
| Literature: Berardi; Colabufo; Giudice; Perrone; Tortorella; Govoni; Lucchi Journal of Medicinal Chemistry, 1996 , vol. 39, # 1 p. 176 - 182 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
A new method for evaluating sigma(2) ligand activity in the isolated guinea-pig bladder.
Naunyn Schmiedebergs Arch. Pharmacol. 368 , 106-112, (2003) We demonstrated the presence of sigma(2) receptors in the guinea-pig ileum by saturation analysis and extended our investigation to guinea-pig bladder and rat bladder. In functional assays of the isol... |
| 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine,dihydrochloride |