6-Chloro-1H-indole
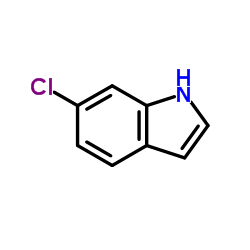
6-Chloro-1H-indole structure
|
Common Name | 6-Chloro-1H-indole | ||
|---|---|---|---|---|
| CAS Number | 17422-33-2 | Molecular Weight | 151.59 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 293.0±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H6ClN | Melting Point | 87-90 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 158.9±5.4 °C | |
Use of 6-Chloro-1H-indole6-Chloroindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 6-Chloroindole |
|---|---|
| Synonym | More Synonyms |
| Description | 6-Chloroindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 产品规格6-氯吲哚;6-氯-1H-吲哚; |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 293.0±13.0 °C at 760 mmHg |
| Melting Point | 87-90 °C(lit.) |
| Molecular Formula | C8H6ClN |
| Molecular Weight | 151.59 |
| Flash Point | 158.9±5.4 °C |
| Exact Mass | 151.018875 |
| PSA | 15.79000 |
| LogP | 2.74 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.688 |
| InChIKey | YTYIMDRWPTUAHP-UHFFFAOYSA-N |
| SMILES | Clc1ccc2cc[nH]c2c1 |
| Stability | Store in Refrigerator |
| Water Solubility | slightly soluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25-S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Design, synthesis and structure-activity relationship studies of novel and diverse cyclooxygenase-2 inhibitors as anti-inflammatory drugs.
J. Enzyme Inhib. Med. Chem. 29(6) , 846-67, (2014) Because of the pivotal role of cyclooxygenase (COX) in the inflammatory processes, non-steroidal anti-inflammatory drugs (NSAIDs) that suppress COX activities have been used clinically for the treatme... |
|
|
Toward prediction of alkane/water partition coefficients.
J. Med. Chem. 51 , 3720-30, (2008) Partition coefficients were measured for 47 compounds in the hexadecane/water ( P hxd) and 1-octanol/water ( P oct) systems. Some types of hydrogen bond acceptor presented by these compounds to the pa... |
|
|
Production of substituted L-tryptophans by fermentation.
Appl. Microbiol. 21(5) , 841-3, (1971) Claviceps purpurea has been shown to produce extracellular l-tryptophan from indole in stirred fermentors. The substrate specificity of this conversion was investigated by using substituted indoles, a... |
| 6-chloro indole |
| 1H-Indole, 6-chloro- |
| MFCD00005681 |
| 6-Chloroindole |
| 6-Cl-indole |
| EINECS 241-449-4 |
| 1H-Indole,6-chloro |
| 6-chlorolindole |
| 6-Chloro-1H-indole |
 CAS#:123-75-1
CAS#:123-75-1 CAS#:89-59-8
CAS#:89-59-8 CAS#:4637-24-5
CAS#:4637-24-5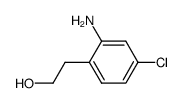 CAS#:124043-86-3
CAS#:124043-86-3![5-chloro-trans-2-[β-(dimethylamino)vinyl]-nitrobenzene Structure](https://image.chemsrc.com/caspic/089/32989-56-3.png) CAS#:32989-56-3
CAS#:32989-56-3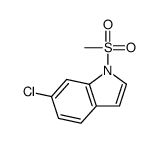 CAS#:88131-68-4
CAS#:88131-68-4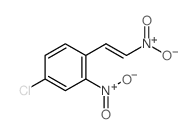 CAS#:90001-58-4
CAS#:90001-58-4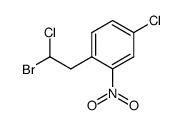 CAS#:85355-58-4
CAS#:85355-58-4 CAS#:106851-31-4
CAS#:106851-31-4 CAS#:33468-35-8
CAS#:33468-35-8 CAS#:50517-12-9
CAS#:50517-12-9 CAS#:50517-10-7
CAS#:50517-10-7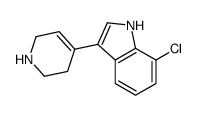 CAS#:180160-69-4
CAS#:180160-69-4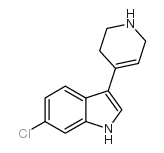 CAS#:180160-77-4
CAS#:180160-77-4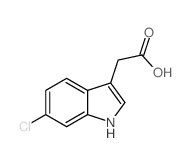 CAS#:1912-44-3
CAS#:1912-44-3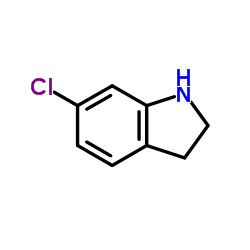 CAS#:52537-00-5
CAS#:52537-00-5 CAS#:703-82-2
CAS#:703-82-2 CAS#:766557-02-2
CAS#:766557-02-2
