Apicidin
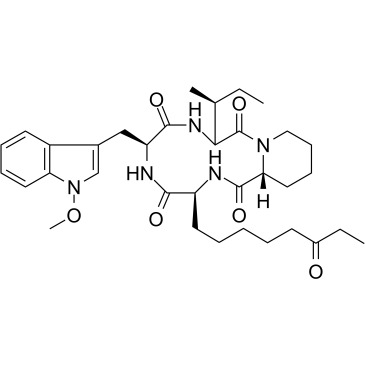
Apicidin structure
|
Common Name | Apicidin | ||
|---|---|---|---|---|
| CAS Number | 183506-66-3 | Molecular Weight | 609.75600 | |
| Density | 1.27g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C34H49N5O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of ApicidinApicidin (OSI 2040) is a fungal metabolite, acts as a histone deacetylase (HDAC) inhibitor, with antiparasitic activity and a broad spectrum antiproliferative activity[1]. |
| Name | (3S,6S,9S,12R)-3-[(2S)-butan-2-yl]-6-[(1-methoxyindol-3-yl)methyl]-9-(6-oxooctyl)-1,4,7,10-tetrazabicyclo[10.4.0]hexadecane-2,5,8,11-tetrone |
|---|---|
| Synonym | More Synonyms |
| Description | Apicidin (OSI 2040) is a fungal metabolite, acts as a histone deacetylase (HDAC) inhibitor, with antiparasitic activity and a broad spectrum antiproliferative activity[1]. |
|---|---|
| Related Catalog | |
| Target |
HDAC[1] |
| References |
| Density | 1.27g/cm3 |
|---|---|
| Molecular Formula | C34H49N5O6 |
| Molecular Weight | 609.75600 |
| Exact Mass | 609.35300 |
| PSA | 138.84000 |
| LogP | 4.12130 |
| Index of Refraction | 1.615 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H310-H330 |
| Precautionary Statements | P260-P264-P280-P284-P302 + P350-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic; |
| Risk Phrases | R26/27/28 |
| Safety Phrases | 22-26-36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 2 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
|
Epigenetic reprogramming of the type III interferon response potentiates antiviral activity and suppresses tumor growth.
PLoS Biol. 12(1) , e1001758, (2014) Type III interferon (IFN-λ) exhibits potent antiviral activity similar to IFN-α/β, but in contrast to the ubiquitous expression of the IFN-α/β receptor, the IFN-λ receptor is restricted to cells of ep... |
|
|
TGF-β1, but not bone morphogenetic proteins, activates Smad1/5 pathway in primary human macrophages and induces expression of proatherogenic genes.
J. Immunol. 194(2) , 709-18, (2015) Macrophages are responsible for the control of inflammation and healing, and their malfunction results in cardiometabolic disorders. TGF-β is a pleiotropic growth factor with dual (protective and detr... |
|
|
Determination of the Mycotoxin Content in Distiller's Dried Grain with Solubles Using a Multianalyte UHPLC-MS/MS Method.
J. Agric. Food Chem. 63 , 9441-51, (2015) There are more than 300 potential mycotoxins that can contaminate food and feed and cause adverse effects in humans and animals. The data on the co-occurrence of mycotoxins in novel animal feed materi... |
| Acipidin |
| (3S,6S,9S,15aR)-9-[(2S)-Butan-2-yl]-6-[(1-methoxy-1H-indol-3-yl)methyl]-3-(6-oxooctyl)octahydro-2H-pyrido[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10(3H,12H)-tetrone |
| 2H-Pyrido[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10(3H,12H)-tetrone, octahydro-6-[(1-methoxy-1H-indol-3-yl)methyl]-9-[(1S)-1-methylpropyl]-3-(6-oxooctyl)-, (3S,6S,9S,15aR)- |
| OSI-2040 |
| (3S,6S,9S,15aR)-9-(butan-2-yl)-6-[(1-methoxy-1H-indol-3-yl)methyl]-3-(6-oxooctyl)octahydro-2H-pyrido[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10(3H,12H)-tetrone |
| (3S,6S,9S,15aR)-9-[(2S)-2-Butanyl]-6-[(1-methoxy-1H-indol-3-yl)methyl]-3-(6-oxooctyl)octahydro-2H-pyrido[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10(3H,12H)-tetrone |
| Apicidin |
 CAS#:111-29-5
CAS#:111-29-5 CAS#:13139-16-7
CAS#:13139-16-7 CAS#:83067-20-3
CAS#:83067-20-3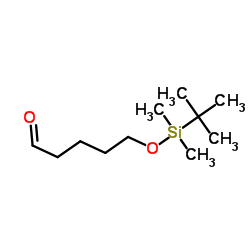 CAS#:87184-80-3
CAS#:87184-80-3![(S)-O-[(2,2-DIMETHYL-1,3-DIOXOLAN-4-YL)METHYL]-HYDROXYAMINEHYDROHLORIDE Structure](https://image.chemsrc.com/caspic/096/35677-83-9.png) CAS#:35677-83-9
CAS#:35677-83-9 CAS#:330814-50-1
CAS#:330814-50-1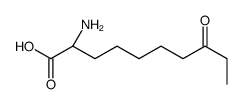 CAS#:375858-14-3
CAS#:375858-14-3 CAS#:73-32-5
CAS#:73-32-5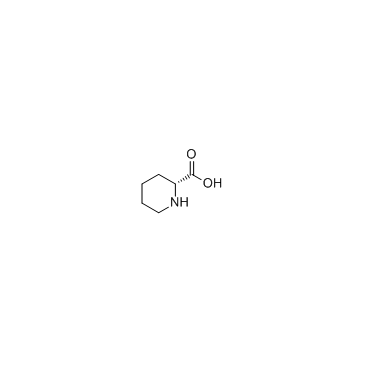 CAS#:1723-00-8
CAS#:1723-00-8 CAS#:73-22-3
CAS#:73-22-3