PD-161570
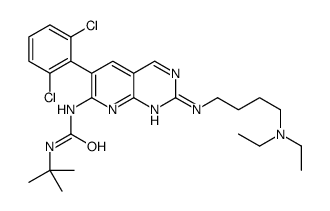
PD-161570 structure
|
Common Name | PD-161570 | ||
|---|---|---|---|---|
| CAS Number | 192705-80-9 | Molecular Weight | 532.50800 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C26H35Cl2N7O | Melting Point | 157 °C | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of PD-161570PD-161570 is a potent and ATP-competitive human FGF-1 receptor inhibitor with an IC50 of 39.9 nM and a Ki of 42 nM. PD-161570 also inhibits the PDGFR, EGFR and c-Src tyrosine kinases with IC50 values of 310 nM, 240 nM, and 44 nM, respectively. PD-161570 inhibits PDGF-stimulated autophosphorylation and FGF-1 receptor phosphorylation with IC50s of 450 nM and 622 nM, respectively[1][2]. PD-161570 is also a bone morphogenetic proteins (BMPs) and TGF-β signaling inhibitor[3]. |
| Name | 1-tert-butyl-3-[6-(2,6-dichlorophenyl)-2-[4-(diethylamino)butylamino]pyrido[2,3-d]pyrimidin-7-yl]urea |
|---|---|
| Synonym | More Synonyms |
| Description | PD-161570 is a potent and ATP-competitive human FGF-1 receptor inhibitor with an IC50 of 39.9 nM and a Ki of 42 nM. PD-161570 also inhibits the PDGFR, EGFR and c-Src tyrosine kinases with IC50 values of 310 nM, 240 nM, and 44 nM, respectively. PD-161570 inhibits PDGF-stimulated autophosphorylation and FGF-1 receptor phosphorylation with IC50s of 450 nM and 622 nM, respectively[1][2]. PD-161570 is also a bone morphogenetic proteins (BMPs) and TGF-β signaling inhibitor[3]. |
|---|---|
| Related Catalog | |
| Target |
FGFR1:39.9 nM (IC50) FGFR1:42 nM (Ki) FGFR1 autophosphorylation:622 nM (IC50) PDGFRβ:262 nM (IC50) PDGFR:310 nM (IC50) EGFR:240 nM (IC50) c-Src:44 nM (IC50) TGF-β Receptor |
| In Vitro | PD-161570 (Compound 6c; 0.1-1 µM; 1-8 days; VSMCs) treatment inhibits PDGF-stimulated vascular smooth muscle cell proliferation in a dose dependent fashion with an IC50 of 0.3 µM at day 8[1]. PD-161570 suppresses constitutive phosphorylation of the FGF-1 receptor in both human ovarian carcinoma cells (A121(p)) and Sf9 insect cells overexpressing the human FGF-1 receptor and blocked the growth of A121(p) cells in culture[2]. PD-161570 can potent inhibit basic fibroblast growth factor (bFGF)-mediated angiogenesis[4]. Cell Proliferation Assay[1] Cell Line: Vascular smooth muscles cells (VSMCs) Concentration: 0.1 µM, 0.3 µM, 1 µM Incubation Time: 1 day, 3 days, 6 days, 8 days Result: Inhibited VSMC proliferation in a dose dependent fashion with an IC50 of 0.3 µM at day 8. |
| References |
| Melting Point | 157 °C |
|---|---|
| Molecular Formula | C26H35Cl2N7O |
| Molecular Weight | 532.50800 |
| Exact Mass | 531.22800 |
| PSA | 101.79000 |
| LogP | 6.15180 |
| InChIKey | MKVMEJKNLUWFSQ-UHFFFAOYSA-N |
| SMILES | CCN(CC)CCCCNc1ncc2cc(-c3c(Cl)cccc3Cl)c(NC(=O)NC(C)(C)C)nc2n1 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335 |
| Precautionary Statements | P261-P301 + P310-P305 + P351 + P338 |
| RIDADR | UN 2811 6.1 / PGIII |
| 1-TERT-BUTYL-3-[6-(2,6-DICHLOROPHENYL)-2-[[4-(DIETHYLAMINO)BUTYL]AMINO]PYRIDO[2,3-D]PYRIMIDIN-7-YL]UREA |
| 6-arylpyrido[2,3-d]pyrimidine deriv. 33 |
| HMS3262A10 |