S-Allylmercaptocysteine
Modify Date: 2025-08-25 20:04:09
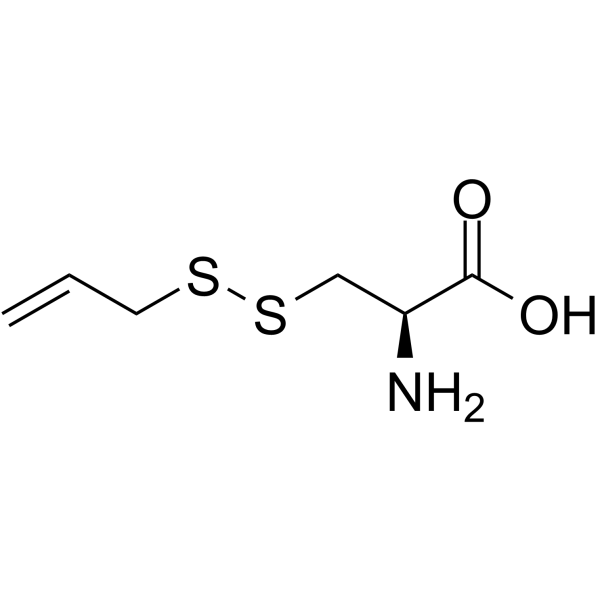
S-Allylmercaptocysteine structure
|
Common Name | S-Allylmercaptocysteine | ||
|---|---|---|---|---|
| CAS Number | 2281-22-3 | Molecular Weight | 193.29 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H11NO2S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of S-AllylmercaptocysteineS-allylmercaptocysteine, an organic sulfur compound extracted from garlic, has anti-inflammatory and anti-oxidative effects for various pulmonary diseases. S-allylmercaptocysteine achieves its anti-cancer effect through a variety of pathways such as inducing the apoptosis of cancer cells through the TGF-β signaling pathway, or reducing the NF-κB activity and up-regulating Nrf2 to achieve the effects of anti-inflammation and anti-oxidation[1][2][3]. |
| Name | S-Allylmercaptocysteine |
|---|
| Description | S-allylmercaptocysteine, an organic sulfur compound extracted from garlic, has anti-inflammatory and anti-oxidative effects for various pulmonary diseases. S-allylmercaptocysteine achieves its anti-cancer effect through a variety of pathways such as inducing the apoptosis of cancer cells through the TGF-β signaling pathway, or reducing the NF-κB activity and up-regulating Nrf2 to achieve the effects of anti-inflammation and anti-oxidation[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | S-Allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation[2]. S-Allylmercaptocysteine (400 μM; 48 hours) induces apoptosis evaluated by detecting the activated caspase 3 and cleaved PARP in SW620, SW480, and Caco-2 cells. Both activated caspase 3 and cleaved PARP1 are found in the cells treated with SAMC while no activated PARP1 and caspase 3 are found in the untreated control cells[4]. |
| In Vivo | S-Allylmercaptocysteine (25 and 50 mg/kg; oral gavage) could significantly ameliorate the pathological structure, and decrease inflammatory cell infiltration and pro-inflammatory cytokines in bronchoalveolar lavage fluid (BALF) in BLM-induced pulmonary fibrosis mice. S-Allylmercaptocysteine shows an anti-fibrosis effect by increasing anti-oxidants like HO-1, GSH and SOD as well as decreasing hydroxyproline (HYP) in BLM-induced mice[1]. |
| References |
| Molecular Formula | C6H11NO2S2 |
|---|---|
| Molecular Weight | 193.29 |
| InChIKey | WYQZZUUUOXNSCS-YFKPBYRVSA-N |
| SMILES | C=CCSSCC(N)C(=O)O |