(R)-(+)-Thalidomide
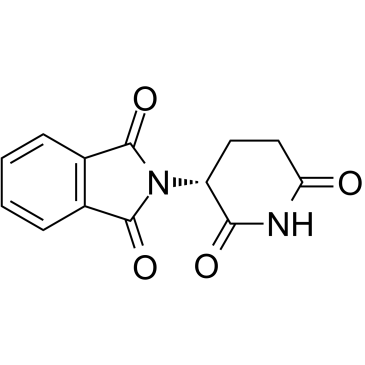
(R)-(+)-Thalidomide structure
|
Common Name | (R)-(+)-Thalidomide | ||
|---|---|---|---|---|
| CAS Number | 2614-06-4 | Molecular Weight | 258.22900 | |
| Density | 1.503g/cm3 | Boiling Point | 509.7ºC at 760 mmHg | |
| Molecular Formula | C13H10N2O4 | Melting Point | 269-271ºC | |
| MSDS | Chinese USA | Flash Point | 262.1ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of (R)-(+)-Thalidomide(R)-Thalidomide ((R)-(+)-Thalidomide) is the R-enantiomer of Thalidomide. (R)-Thalidomide has sedative properties[1][2]. |
| Name | (R)-thalidomide |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-Thalidomide ((R)-(+)-Thalidomide) is the R-enantiomer of Thalidomide. (R)-Thalidomide has sedative properties[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | The transport of the (R)-Thalidomide from the R-imprinted MIP-1 through the donor phase to the receiver phase is much less owing to the stronger retention of the thalidomide in the organic phase. With the affinity of (R)-Thalidomide by the MIP present surface capture, that is more strongly than the other forms. In the case of (R)-Thalidomide, it is found to bind to the selective sites of the MIP more strongly than the other which reflects their different biological entities[1]. The (S)-Thalidomide imprints MIP nanoparticles exerted a greater cytotoxic effect on the caco-2 cells than the (R)-Thalidomide imprinted MIPs[1]. |
| In Vivo | Adult female F344 rats are implanted with 9L gliosarcoma tumours intracranially, subcutaneously (flank), or both. Effectiveness of oral thalidomide alone, and with intraperitoneal BCNU or cisplatin combination chemotherapy, is assessed after several weeks treatment. Both serum and tissue concentrations of (R)-thalidomide are 40-50% greater than those of (S)-thalidomide. Co-administration of BCNU or cisplatin with thalidomide did not alter the concentration enantioselectivity[1]. |
| References |
| Density | 1.503g/cm3 |
|---|---|
| Boiling Point | 509.7ºC at 760 mmHg |
| Melting Point | 269-271ºC |
| Molecular Formula | C13H10N2O4 |
| Molecular Weight | 258.22900 |
| Flash Point | 262.1ºC |
| Exact Mass | 258.06400 |
| PSA | 83.55000 |
| LogP | 0.35450 |
| Vapour Pressure | 1.65E-10mmHg at 25°C |
| Index of Refraction | 1.646 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R61 |
| Safety Phrases | S45;S53;S36/S37/S39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TI4925000 |
| HS Code | 2925190090 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2925190090 |
|---|---|
| Summary | 2925190090 other imides and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Thalidomide is an inhibitor of angiogenesis.
Proc. Natl. Acad. Sci. U. S. A. 91 , 4082, (1994) Thalidomide is a potent teratogen causing dysmelia (stunted limb growth) in humans. We have demonstrated that orally administered thalidomide is an inhibitor of angiogenesis induced by basic fibroblas... |
|
|
Thalidomide inhibits the replication of human immunodeficiency virus type 1.
Proc. Natl. Acad. Sci. U. S. A. 90 , 5974-5878, (1993) Thalidomide, a selective inhibitor of tumor necrosis factor alpha (TNF-alpha) synthesis, suppresses the activation of latent human immunodeficiency virus type 1 (HIV-1) in a monocytoid (U1) line. The ... |
|
|
Thalidomide induces limb anomalies by PTEN stabilization, Akt suppression, and stimulation of caspase-dependent cell death.
Mol. Cell. Biol. 28(2) , 529-538, (2008) Thalidomide, a drug used for the treatment of multiple myeloma and inflammatory diseases, is also a teratogen that causes birth defects, such as limb truncations and microphthalmia, in humans. Thalido... |
| (+)-Thalidomide |
| MFCD00210220 |
| (R)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione |
| (R)-(+)-Thalidomide |
| R-(+)-2-(2,6-Dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione |
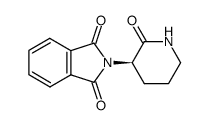 CAS#:771468-60-1
CAS#:771468-60-1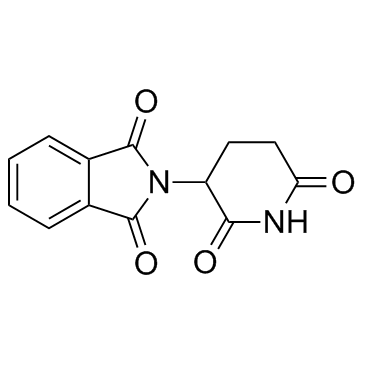 CAS#:50-35-1
CAS#:50-35-1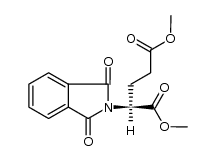 CAS#:35457-98-8
CAS#:35457-98-8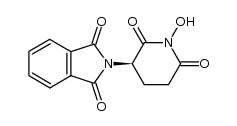 CAS#:170079-56-8
CAS#:170079-56-8 CAS#:22509-74-6
CAS#:22509-74-6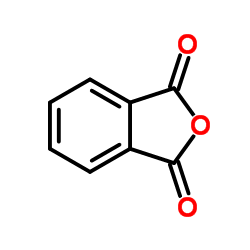 CAS#:85-44-9
CAS#:85-44-9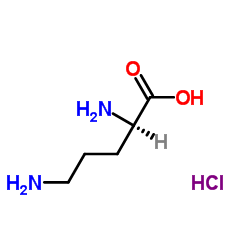 CAS#:16682-12-5
CAS#:16682-12-5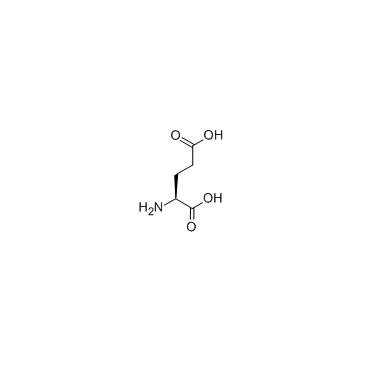 CAS#:56-86-0
CAS#:56-86-0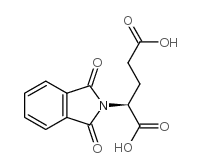 CAS#:340-90-9
CAS#:340-90-9 CAS#:6525-53-7
CAS#:6525-53-7 CAS#:841-67-8
CAS#:841-67-8