(S)-Thalidomide

(S)-Thalidomide structure
|
Common Name | (S)-Thalidomide | ||
|---|---|---|---|---|
| CAS Number | 841-67-8 | Molecular Weight | 258.22900 | |
| Density | 1.503g/cm3 | Boiling Point | 509.7ºC at 760 mmHg | |
| Molecular Formula | C13H10N2O4 | Melting Point | 269-271ºC | |
| MSDS | Chinese USA | Flash Point | 262.1ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of (S)-Thalidomide(S)-Thalidomide ((S)-(-)-Thalidomide) is the S-enantiomer of Thalidomide. (S)-Thalidomide has immunomodulatory, anti-inflammatory, antiangiogenic and pro-apoptotic effects[1][2][3]. (S)-Thalidomide induces teratogenic effects by binding to cereblon (CRBN) [4]. |
| Name | (S)-thalidomide |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-Thalidomide ((S)-(-)-Thalidomide) is the S-enantiomer of Thalidomide. (S)-Thalidomide has immunomodulatory, anti-inflammatory, antiangiogenic and pro-apoptotic effects[1][2][3]. (S)-Thalidomide induces teratogenic effects by binding to cereblon (CRBN) [4]. |
|---|---|
| Related Catalog | |
| Target |
Apoptosis[1] |
| In Vitro | (S)-Thalidomide treatment results in a reduction in cell viability in U266 cells with an IC50 of 362 μM[1]. (S)-Thalidomide treatment increased apoptosis in a dose-dependent manner in U266 cells[1]. There are changes in the expression profile of genes involved in angiogenesis and apoptosis, but the changes are most dramatic in the apoptotic genes. In particular, the expression of I-κB kinase is decreased by two-fold, which is associated with a four-fold decrease in NF-κB expression. (S)-Thalidomide increases the Bax:Bcl-2 ratio, also increases I-kB protein levels, and decreases NF-kB activity. A dramatic decrease in Bcl-2 expression with (S)-Thalidomide suggests a possible enhancement of cytotoxic effect if combined with other cytotoxic agents[1]. Cell Viability Assay[1] Cell Line: U266 MM cells Concentration: 0 µM, 10 µM, 100 µM, 150 µM, 200 µM, 1000 µM Incubation Time: 3 days Result: A reduction in cell viability was observed in U266 cells. Apoptosis Analysis[1] Cell Line: U266 MM cells Concentration: 100 µM, 150 µM, 200 µM, 1000 µM Incubation Time: 3 days Result: Increased apoptosis in U266 cells. |
| In Vivo | Thalidomide does cause limb reduction defects in chick embryos as long as the embryos are directly exposed to the drug. The most useful techniques are implanting Thalidomide-soaked beads into the embryo immediately adjacent to the limb territory or soaking presumptive chick limb territories in Thalidomide and then grafting the explants to a host embryo celom. Thalidomide affects the chick limb grafted to a host embryo in a dose response fashion. Furthermore, (S)-Thalidomide is more teratogenic than (R)-Thalidomide[1]. |
| References |
| Density | 1.503g/cm3 |
|---|---|
| Boiling Point | 509.7ºC at 760 mmHg |
| Melting Point | 269-271ºC |
| Molecular Formula | C13H10N2O4 |
| Molecular Weight | 258.22900 |
| Flash Point | 262.1ºC |
| Exact Mass | 258.06400 |
| PSA | 83.55000 |
| LogP | 0.35450 |
| Index of Refraction | 1.646 |
| InChIKey | UEJJHQNACJXSKW-VIFPVBQESA-N |
| SMILES | O=C1CCC(N2C(=O)c3ccccc3C2=O)C(=O)N1 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R61 |
| Safety Phrases | 53-36/37/39-45 |
| RIDADR | UN 2811 6.1/PG 2 |
| RTECS | TI4925050 |
| HS Code | 2925190090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2925190090 |
|---|---|
| Summary | 2925190090 other imides and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Thalidomide is an inhibitor of angiogenesis.
Proc. Natl. Acad. Sci. U. S. A. 91 , 4082, (1994) Thalidomide is a potent teratogen causing dysmelia (stunted limb growth) in humans. We have demonstrated that orally administered thalidomide is an inhibitor of angiogenesis induced by basic fibroblas... |
|
|
Thalidomide inhibits the replication of human immunodeficiency virus type 1.
Proc. Natl. Acad. Sci. U. S. A. 90 , 5974-5878, (1993) Thalidomide, a selective inhibitor of tumor necrosis factor alpha (TNF-alpha) synthesis, suppresses the activation of latent human immunodeficiency virus type 1 (HIV-1) in a monocytoid (U1) line. The ... |
|
|
Thalidomide induces limb anomalies by PTEN stabilization, Akt suppression, and stimulation of caspase-dependent cell death.
Mol. Cell. Biol. 28(2) , 529-538, (2008) Thalidomide, a drug used for the treatment of multiple myeloma and inflammatory diseases, is also a teratogen that causes birth defects, such as limb truncations and microphthalmia, in humans. Thalido... |
| (-)-N-[(S)-2,6-Dioxo-3-piperidinyl]phthalimide |
| (-)-Thalidomide |
| 6-dioxo-3-piperidyl)-n-(l-(-)-phthalimid |
| INHIBITOR OF ANGIOG EN |
| S(-)-2-(2,6-DIOXO-3-PIPERIDINYL)-1H-ISOINDOLE-1,3(2H)-DIONE |
| (3S)-3-(1,3-Dioxo-2H-isoindole-2-yl)piperidine-2,6-dione |
| 1H-Isoindole-1,3(2H)-dione,2-[(3S)-2,6-dioxo-3-piperidinyl]- |
| (-)-THALIDOMIDE &N-[(S)-2,6-Dioxopiperidine-3-yl]phthalimide |
| L-thalidomide |
| 6-dioxo-3-piperidinyl)-3(2h)-dion(s)-1h-isoindole-2-(2 |
| 2-[(3S)-2,6-Dioxo-3-piperidyl]-1H-isoindole-1,3(2H)-dione |
| (-)-(S)-1,3-dioxo-2-(2',6'-dioxopiperidin-3'-yl)isoindol |
| MFCD00210219 |
 CAS#:3343-29-1
CAS#:3343-29-1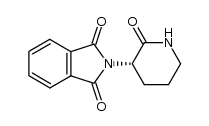 CAS#:61071-65-6
CAS#:61071-65-6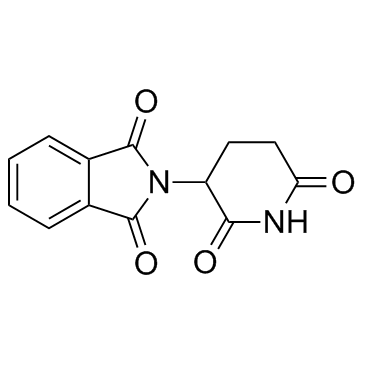 CAS#:50-35-1
CAS#:50-35-1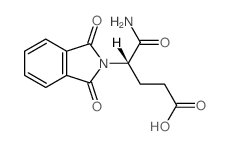 CAS#:2614-08-6
CAS#:2614-08-6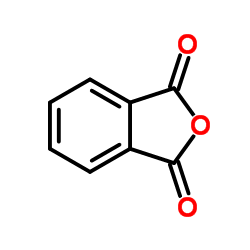 CAS#:85-44-9
CAS#:85-44-9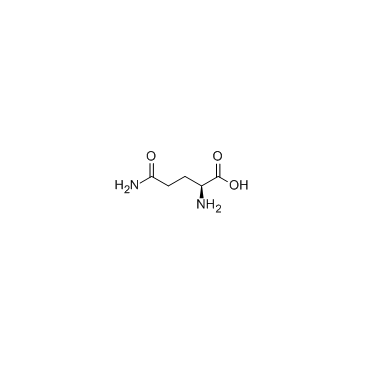 CAS#:56-85-9
CAS#:56-85-9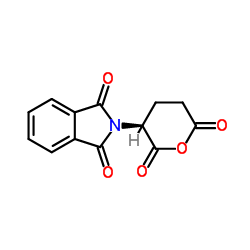 CAS#:25830-77-7
CAS#:25830-77-7 CAS#:22509-74-6
CAS#:22509-74-6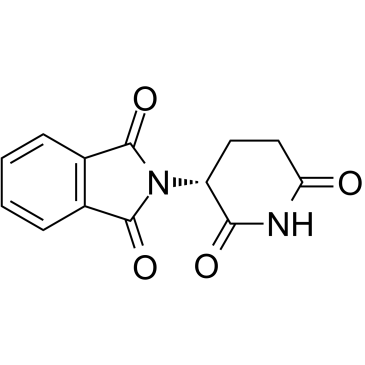 CAS#:2614-06-4
CAS#:2614-06-4