L-glutamic acid
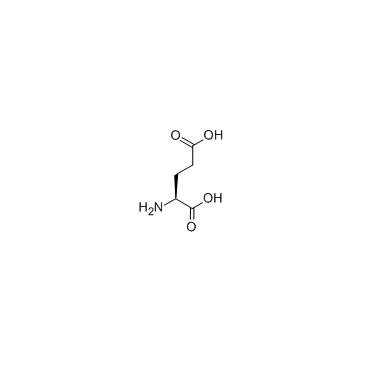
L-glutamic acid structure
|
Common Name | L-glutamic acid | ||
|---|---|---|---|---|
| CAS Number | 56-86-0 | Molecular Weight | 147.129 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 333.8±32.0 °C at 760 mmHg | |
| Molecular Formula | C5H9NO4 | Melting Point | 205 °C (dec.)(lit.) | |
| MSDS | USA | Flash Point | 155.7±25.1 °C | |
Use of L-glutamic acid(S)-Glutamic acid acts as an excitatory transmitter, shows a direct activating effect on the release of DA from dopaminergic terminals. |
| Name | L-glutamic acid |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-Glutamic acid acts as an excitatory transmitter, shows a direct activating effect on the release of DA from dopaminergic terminals. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite DA |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 333.8±32.0 °C at 760 mmHg |
| Melting Point | 205 °C (dec.)(lit.) |
| Molecular Formula | C5H9NO4 |
| Molecular Weight | 147.129 |
| Flash Point | 155.7±25.1 °C |
| Exact Mass | 147.053162 |
| PSA | 100.62000 |
| LogP | -1.43 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.522 |
| Water Solubility | 7.5 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | LZ9700000 |
| HS Code | 2922421000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922421000 |
|---|---|
| Summary | HS: 2922421000. (s)-2-aminopentanedioic acid. VAT:17.0%. tax rebate rate:9.0%. supervision conditions:ab(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tarrif:10.0%. general tariff:90.0% |
|
Rad23 interaction with the proteasome is regulated by phosphorylation of its ubiquitin-like (UbL) domain.
J. Mol. Biol. 426(24) , 4049-60, (2014) Rad23 was identified as a DNA repair protein, although a role in protein degradation has been described. The protein degradation function of Rad23 contributes to cell cycle progression, stress respons... |
|
|
EAAT2 (GLT-1; slc1a2) glutamate transporters reconstituted in liposomes argues against heteroexchange being substantially faster than net uptake.
J. Neurosci. 34(40) , 13472-85, (2014) The EAAT2 glutamate transporter, accounts for >90% of hippocampal glutamate uptake. Although EAAT2 is predominantly expressed in astrocytes, ∼10% of EAAT2 molecules are found in axon terminals. Despit... |
|
|
Apoptotic and neurotoxic actions of 4-para-nonylphenol are accompanied by activation of retinoid X receptor and impairment of classical estrogen receptor signaling
J. Steroid Biochem. Mol. Biol. 144 Pt B , 334-47, (2014) • Age-dependent action of nonylphenol (NP) on mouse neuronal cells was demonstrated. • Estrogen receptor (ER) antagonists potentiated the NP-induced apoptosis and toxicity. • ER agonists inhibited the... |
| L-Glutamic acid |
| Glusate |
| H-L-GLU-OH |
| L-GLU |
| H-GLU-OH |
| GLU |
| L-Glutamicacid |
| GLUTACID |
| Aciglut |
| EINECS 200-293-7 |
| MFCD00002634 |
| glutamic |
| Glutaton |
| (S)-Glutamic acid |
 CAS#:91871-28-2
CAS#:91871-28-2 CAS#:5680-86-4
CAS#:5680-86-4 CAS#:1676-73-9
CAS#:1676-73-9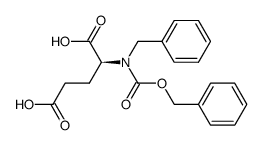 CAS#:132316-96-2
CAS#:132316-96-2![(5S)-1-tert-butoxycarbonyl-5-(5-methyl-2,7,8-trioxabicyclo[3.2.1]oct-1-yl)-5H-pyrrolin-2-one Structure](https://image.chemsrc.com/caspic/428/570429-70-8.png) CAS#:570429-70-8
CAS#:570429-70-8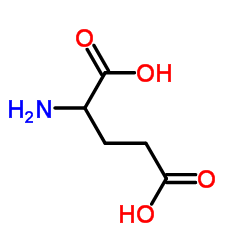 CAS#:617-65-2
CAS#:617-65-2![3-[3-((allyloxy)carbonyl)-5-oxo-1,3-oxazolan-4-yl]propanoic acid Structure](https://image.chemsrc.com/caspic/069/138965-46-5.png) CAS#:138965-46-5
CAS#:138965-46-5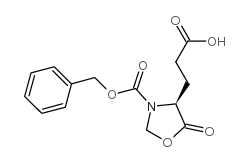 CAS#:23632-67-9
CAS#:23632-67-9 CAS#:71989-18-9
CAS#:71989-18-9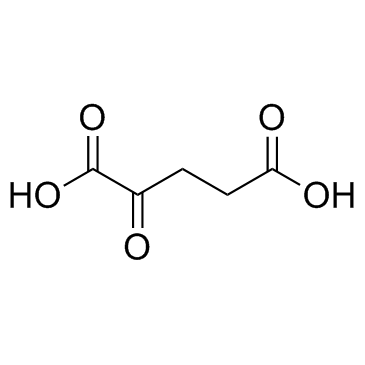 CAS#:328-50-7
CAS#:328-50-7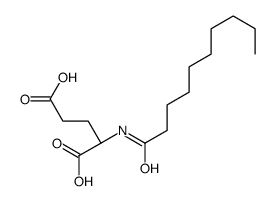 CAS#:111276-71-2
CAS#:111276-71-2 CAS#:124-40-3
CAS#:124-40-3 CAS#:74-89-5
CAS#:74-89-5 CAS#:75-50-3
CAS#:75-50-3![(5S)-5-[[[(tert-Butyl)dimethylsilyl]oxy]methyl]-2-pyrrolidinone structure](https://image.chemsrc.com/caspic/052/106191-02-0.png) CAS#:106191-02-0
CAS#:106191-02-0![(2S)-2-[[5-(dimethylamino)naphthalen-1-yl]sulfonylamino]pentanedioic acid structure](https://image.chemsrc.com/caspic/277/1101-68-4.png) CAS#:1101-68-4
CAS#:1101-68-4 CAS#:45214-91-3
CAS#:45214-91-3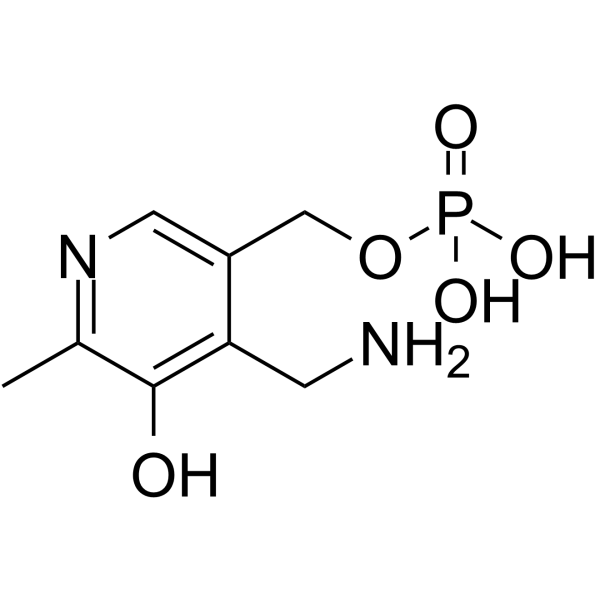 CAS#:529-96-4
CAS#:529-96-4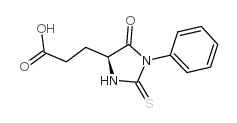 CAS#:5624-27-1
CAS#:5624-27-1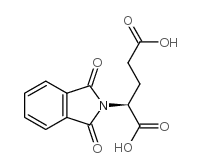 CAS#:340-90-9
CAS#:340-90-9
