Pectolinarin

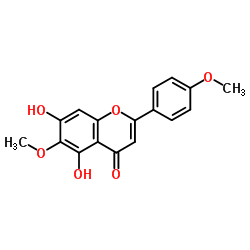
Pectolinarin structure
|
Common Name | Pectolinarin | ||
|---|---|---|---|---|
| CAS Number | 28978-02-1 | Molecular Weight | 622.571 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 896.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C29H34O15 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 292.4±27.8 °C | |
Use of PectolinarinPectolinarin, isolated from Cirsium chanroenicum, possesses anti-inflammatory activity[1]. Pectolinarin inhibits secretion of IL-6 and IL-8, as well as the production of PGE2 and NO. Pectolinarin suppresses cell proliferation and inflammatory response and induces apoptosis via inactivation of the PI3K/Akt pathway[2]. |
| Name | Pectolinarin |
|---|---|
| Synonym | More Synonyms |
| Description | Pectolinarin, isolated from Cirsium chanroenicum, possesses anti-inflammatory activity[1]. Pectolinarin inhibits secretion of IL-6 and IL-8, as well as the production of PGE2 and NO. Pectolinarin suppresses cell proliferation and inflammatory response and induces apoptosis via inactivation of the PI3K/Akt pathway[2]. |
|---|---|
| Related Catalog | |
| Target |
IL-6 IL-8 PGE2 NO Apoptosis |
| In Vitro | Pectolinarin increases Bax level, and decreases Bcl-2 level in rheumatoid arthritis fibroblast-like synoviocytes (RA-FLSs). Pectolinarin inactivates the PI3K/Akt pathway in RA-FLSs[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 896.4±65.0 °C at 760 mmHg |
| Molecular Formula | C29H34O15 |
| Molecular Weight | 622.571 |
| Flash Point | 292.4±27.8 °C |
| Exact Mass | 622.189758 |
| PSA | 227.20000 |
| LogP | 1.71 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.683 |
| InChIKey | DUXQKCCELUKXOE-CBBZIXHGSA-N |
| SMILES | COc1ccc(-c2cc(=O)c3c(O)c(OC)c(OC4OC(COC5OC(C)C(O)C(O)C5O)C(O)C(O)C4O)cc3o2)cc1 |
| Storage condition | 2~8℃ |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 29389090 |
|
~% 
Pectolinarin CAS#:28978-02-1 |
| Literature: Zemplen; Bognar Chemische Berichte, 1941 , vol. 74, p. 1818,1821 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| 5-Hydroxy-6-methoxy-2-(4-methoxyphenyl)-4-oxo-4H-chromen-7-yl 6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranoside |
| 5-hydroxy-6-methoxy-2-(4-methoxyphenyl) |
| 5-Hydroxy-6-methoxy-2-(4-methoxyphenyl)-7-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one |
| neolinarin |
| Pectolinaroside |
| 4H-1-Benzopyran-4-one, 7-((6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl)oxy)-5-hydroxy-6-methoxy-2-(4-methoxyphenyl)- |
| Pectolinarigenin 7-Rutinoside |
| PECTOLINARIN(P) |
| 7-((6-O-(6-Deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl)oxy)-5-hydroxy-6-methoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one |
| Pectolinarin |
| 4H-1-Benzopyran-4-one, 7-[[6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-5-hydroxy-6-methoxy-2-(4-methoxyphenyl)- |
| pectolinarigenin 7-O-rutinoside |
 CAS#:520-12-7
CAS#:520-12-7
