Arecoline hydrobromide
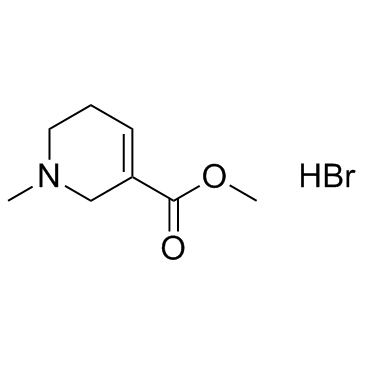
Arecoline hydrobromide structure
|
Common Name | Arecoline hydrobromide | ||
|---|---|---|---|---|
| CAS Number | 300-08-3 | Molecular Weight | 236.106 | |
| Density | N/A | Boiling Point | 209ºC at 760mmHg | |
| Molecular Formula | C8H14BrNO2 | Melting Point | 171-175 °C | |
| MSDS | Chinese USA | Flash Point | 81.1ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Arecoline hydrobromideArecoline Hydrobromide is a muscarinic acetylcholine receptor agonist. Target: mAChRArecoline is an alkaloid found in the areca nut. Arecoline. a drug obtained from the Areca Catechu L., induced a dose-dependent antinociception (0.3-1 mg kg(-1) i.p.) which was prevented by the muscarinic antagonists pirenzepine (0.1 microg per mouse i.c.v.) and S-(-)-ET-126 (0.01 microg per mouse i.c.v.) [1]. Arecoline exerts its excitatory actions by binding to M2-muscarinic receptors on the cell membrane of neurons of the locus coeruleus [2]. Arecoline (1 nM - 1 microM) produced a concentration-dependent contraction in both the longitudinal and the circular smooth muscle of rabbit colon. Atropine (10 microM) abolished the arecoline (80 nM)--induced contraction. M3 receptor antagonist, 4 - DAMP (0.4 microM), abolished the arecoline (80 nM)--related response, whereas M2 receptor antagonist, gallamine (0.4 microM), did not affect the effect of arecoline. These results suggest that arecoline excites the colonic motility via M3 receptor in rabbits [3]. |
| Name | methyl 1-methyl-3,6-dihydro-2H-pyridine-5-carboxylate,hydrobromide |
|---|---|
| Synonym | More Synonyms |
| Description | Arecoline Hydrobromide is a muscarinic acetylcholine receptor agonist. Target: mAChRArecoline is an alkaloid found in the areca nut. Arecoline. a drug obtained from the Areca Catechu L., induced a dose-dependent antinociception (0.3-1 mg kg(-1) i.p.) which was prevented by the muscarinic antagonists pirenzepine (0.1 microg per mouse i.c.v.) and S-(-)-ET-126 (0.01 microg per mouse i.c.v.) [1]. Arecoline exerts its excitatory actions by binding to M2-muscarinic receptors on the cell membrane of neurons of the locus coeruleus [2]. Arecoline (1 nM - 1 microM) produced a concentration-dependent contraction in both the longitudinal and the circular smooth muscle of rabbit colon. Atropine (10 microM) abolished the arecoline (80 nM)--induced contraction. M3 receptor antagonist, 4 - DAMP (0.4 microM), abolished the arecoline (80 nM)--related response, whereas M2 receptor antagonist, gallamine (0.4 microM), did not affect the effect of arecoline. These results suggest that arecoline excites the colonic motility via M3 receptor in rabbits [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 209ºC at 760mmHg |
|---|---|
| Melting Point | 171-175 °C |
| Molecular Formula | C8H14BrNO2 |
| Molecular Weight | 236.106 |
| Flash Point | 81.1ºC |
| Exact Mass | 235.020782 |
| PSA | 29.54000 |
| LogP | 1.31730 |
| Storage condition | Refrigerator |
| Water Solubility | H2O: 0.1 g/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S45-S38-S36/37/39-S28A |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QT2275000 |
| HS Code | 29399990 |
| Precursor 0 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Determination and pharmacokinetic studies of arecoline in dog plasma by liquid chromatography-tandem mass spectrometry.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 969 , 12-8, (2014) A rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed and validated for the determination of arecoline concentration in dog plasma. Plas... |
|
|
Arecoline is cytotoxic for human endothelial cells.
J. Oral. Pathol. Med. 43(10) , 761-9, (2014) Oral submucous fibrosis is a pre-malignant fibrotic condition caused by areca nut use and involves reduced mucosal vascularity. Arecoline is the principal areca nut alkaloid and is cytotoxic for epith... |
| 1-Methyl-1,2,5,6-tetrahydro-pyridin-3-carbonsaeure-methylester,Hydrobromid |
| MFCD00039041 |
| Methyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate hydrobromide (1:1) |
| EINECS 206-087-3 |
| Arecaidine methyl ester hydrobromide |
| methyl 1-methyl-1,2,5,6-tetrahydropyridine-3-carboxylate hydrobromide |
| 3-Pyridinecarboxylic acid, 1,2,5,6-tetrahydro-1-methyl-, methyl ester, hydrobromide (1:1) |
| Arecoline |
| Methyl 1-methyl-1,2,5,6-tetrahydro-3-pyridinecarboxylate hydrobromide |
| ARECOLINE HBr |
| Methyl 1,2,5,6-Tetrahydro-1-methylnicotinate Hydrobromide |
| Methyl-1-methyl-1,2,5,6-tetrahydro-3-pyridincarboxylathydrobromid |
| 1-methyl-1,2,5,6-tetrahydro-pyridine-3-carboxylic acid methyl ester,hydrobromide |
| arecolinium bromide |
| Methyl 1-methyl-1,2,5,6-tetrahydro-3-pyridinecarboxylate hydrobromide (1:1) |
| Arecoline hydrobromide |
| Taeniolin |
| Arecoline bromide |
| 1,2,5,6-Tetrahydro-1-methyl-3-pyridinecarboxylic Acid Methyl Ester Hydrobromide |
| 3-Pyridinecarboxylic acid, 1,2,5,6-tetrahydro-1-methyl-, methyl ester, hydrobromide (9CI) |
| 1-Méthyl-1,2,5,6-tétrahydro-3-pyridinecarboxylate de méthyle bromhydrate |
| Arecoline (hydrobromide) |
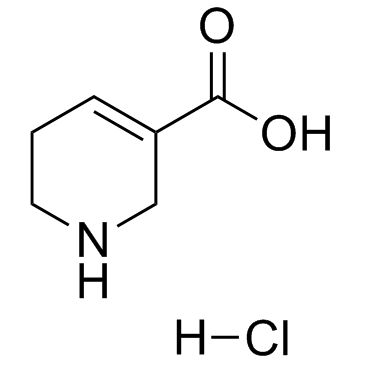 CAS#:6027-91-4
CAS#:6027-91-4 CAS#:498-96-4
CAS#:498-96-4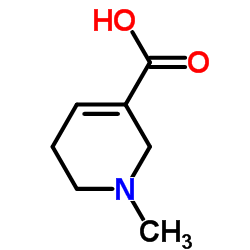 CAS#:499-04-7
CAS#:499-04-7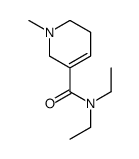 CAS#:26070-52-0
CAS#:26070-52-0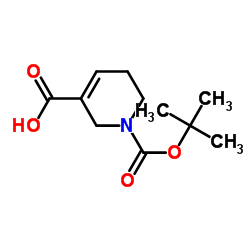 CAS#:86447-11-2
CAS#:86447-11-2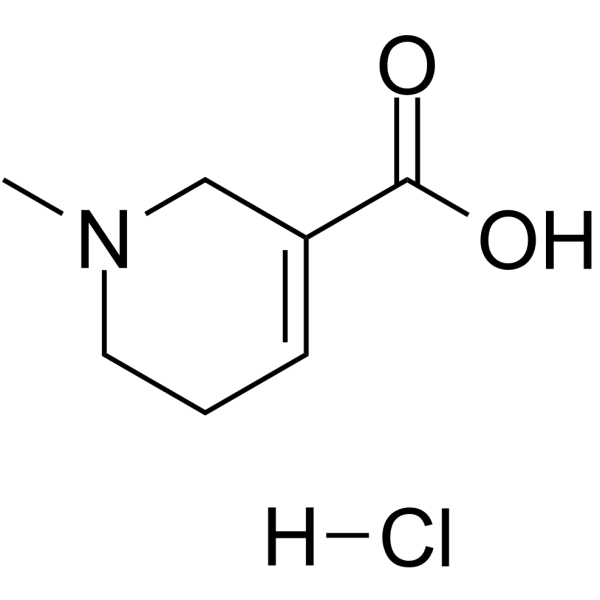 CAS#:6018-28-6
CAS#:6018-28-6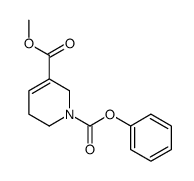 CAS#:323201-17-8
CAS#:323201-17-8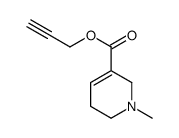 CAS#:35516-99-5
CAS#:35516-99-5 CAS#:115103-54-3
CAS#:115103-54-3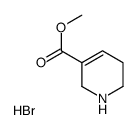 CAS#:17210-51-4
CAS#:17210-51-4
