N-Boc-4-iodopiperidine

N-Boc-4-iodopiperidine structure
|
Common Name | N-Boc-4-iodopiperidine | ||
|---|---|---|---|---|
| CAS Number | 301673-14-3 | Molecular Weight | 311.16 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 318.8±35.0 °C at 760 mmHg | |
| Molecular Formula | C10H18INO2 | Melting Point | 35-38ºC | |
| MSDS | USA | Flash Point | 146.6±25.9 °C | |
Use of N-Boc-4-iodopiperidinetert-Butyl 4-iodopiperidine-1-carboxylate is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | tert-butyl 4-iodopiperidine-1-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | tert-Butyl 4-iodopiperidine-1-carboxylate is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 用于铁和钴催化氮杂环丁烷、吡咯烷和哌啶与格氏试剂的偶联反应。 |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 318.8±35.0 °C at 760 mmHg |
| Melting Point | 35-38ºC |
| Molecular Formula | C10H18INO2 |
| Molecular Weight | 311.16 |
| Flash Point | 146.6±25.9 °C |
| Exact Mass | 311.038208 |
| PSA | 29.54000 |
| LogP | 2.64 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.542 |
| InChIKey | YFWQFKUQVJNPKP-UHFFFAOYSA-N |
| SMILES | CC(C)(C)OC(=O)N1CCC(I)CC1 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933399090 |
|
~91% 
N-Boc-4-iodopip... CAS#:301673-14-3 |
| Literature: Barta, Thomas E.; Becker, Daniel P.; Bedell, Louis J.; Boehm, Terri L.; Brown, David L.; Carroll, Jeffery N.; Chen, Yiyuan; Fobian, Yvette M.; Freskos, John N.; Gasiecki, Alan F.; Grapperhaus, Margaret L.; Heintz, Robert M.; Hockerman, Susan L.; Kassab, Darren J.; Khanna, Ish K.; Kolodziej, Stephen A.; Massa, Mark A.; McDonald, Joseph J.; Mischke, Brent V.; Mischke, Deborah A.; Mullins, Patrick B.; Nagy, Mark A.; Norton, Monica B.; Rico, Joseph G.; Schmidt, Michelle A.; Stehle, Nathan W.; Talley, John J.; Vernier, William F.; Villamil, Clara I.; Wang, Lijuan J.; Wynn, Thomas A. Patent: US2005/9838 A1, 2005 ; Location in patent: Page 143 ; US 20050009838 A1 |
|
~72% 
N-Boc-4-iodopip... CAS#:301673-14-3 |
| Literature: Proskelia SAS Patent: US2008/58348 A1, 2008 ; Location in patent: Page/Page column 15 ; |
|
~94% 
N-Boc-4-iodopip... CAS#:301673-14-3 |
| Literature: TECHNION RandD FOUNDATION LTD.; NISNEVICH, Gennady; GANDELMAN, Mark; KULBITSKI, Kseniya Patent: WO2011/154953 A1, 2011 ; Location in patent: Page/Page column 28-30 ; |
|
~% 
N-Boc-4-iodopip... CAS#:301673-14-3 |
| Literature: Synlett, , # 4 p. 379 - 380 |
|
~% 
N-Boc-4-iodopip... CAS#:301673-14-3 |
| Literature: Synlett, , # 4 p. 379 - 380 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| tert-Butyl 4-iodopiperidine-1-carboxylate |
| 1-(tert-Butoxycarbonyl)-4-iodopiperidine |
| 1-tert-butyl 4-iodo-piperidine-1-carboxylate |
| 1-Piperidinecarboxylic acid, 4-iodo-, 1,1-dimethylethyl ester |
| 1-tert-Butoxycarbonyl-4-iodopiperidine |
| N-Boc-4-iodo-piperidine |
| 1-BOC-4-iodo-piperidine |
| 2-Methyl-2-propanyl 4-iodo-1-piperidinecarboxylate |
| 4-iodo-piperidine-1-carboxylic acid tert-butyl ester |
| tert-Butyl 4-iodo-1-piperidinecarboxylate |
| 1-Boc-4-iodopiperidine |
| N-Boc-4-iodopiperidine |
| tert-butyl 4-iodopiperidinecarboxylate |
| 4-iodo-1-(tert-butoxycarbonyl)piperidine |
| 4-Iodo-1-piperidinecarboxylic Acid tert-Butyl Ester |
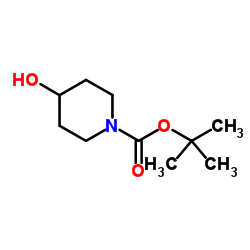
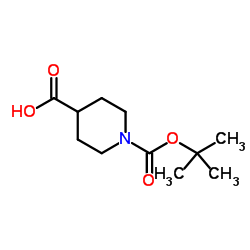

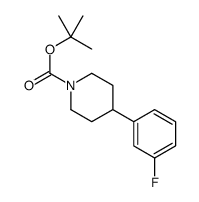 CAS#:1086398-00-6
CAS#:1086398-00-6![3',4',5',6'-tetrahydro-2'H-[2,4'-bipyridine]-1'-carboxylic acid tert-butyl ester structure](https://image.chemsrc.com/caspic/238/206446-49-3.png) CAS#:206446-49-3
CAS#:206446-49-3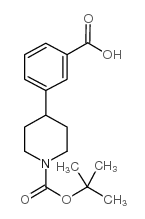 CAS#:828243-30-7
CAS#:828243-30-7 CAS#:123387-49-5
CAS#:123387-49-5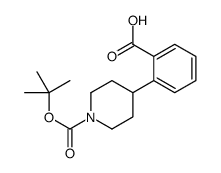 CAS#:170838-26-3
CAS#:170838-26-3 CAS#:170011-56-0
CAS#:170011-56-0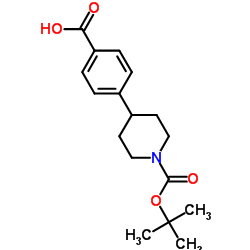 CAS#:149353-75-3
CAS#:149353-75-3 CAS#:193217-39-9
CAS#:193217-39-9 CAS#:162997-34-4
CAS#:162997-34-4 CAS#:1160592-00-6
CAS#:1160592-00-6
