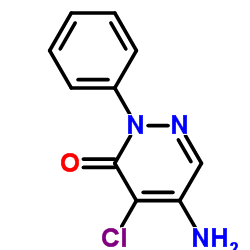
Amezinium methylsulfate
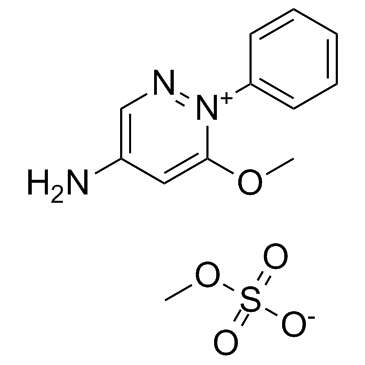
Amezinium methylsulfate structure
|
Common Name | Amezinium methylsulfate | ||
|---|---|---|---|---|
| CAS Number | 30578-37-1 | Molecular Weight | 313.33000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C12H15N3O5S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Amezinium methylsulfateAmezinium metilsulfate has multiple mechanisms, including stimulation of alpha and beta-1 receptors and inhibition ofnoradrenaline and tyramine uptake. Target: alpha and beta-1 receptorsAmezinium metilsulfate is a sympathomimetic drug used for the treatment of low blood pressure. Cardiovascular effects of the new sympathomimetic Amezinium metilsulphate are investigated in 25 patients compared with a control group (n = 25). During spinal/epidural anaesthesia 5 mg amezinium is given i.v. if blood pressure dropped greater than 20 mmHg. from starting-point. A significant recovery of blood pressure (epidural anaesthesia: syst 21%, diast 9%; spinal anaesthesia: syst 13%, diast 6.6%) and a decrease in heart rate (6.8% resp. 4,5%) are thought due to peripheral vasoconstriction. Amezinium proves a stimulating drug for alpha- and beta 1-receptors by stabilising the systemic blood pressure in spinal/epidural anaesthesia. |
| Name | 6-methoxy-1-phenylpyridazin-1-ium-4-amine,methyl sulfate |
|---|---|
| Synonym | More Synonyms |
| Description | Amezinium metilsulfate has multiple mechanisms, including stimulation of alpha and beta-1 receptors and inhibition ofnoradrenaline and tyramine uptake. Target: alpha and beta-1 receptorsAmezinium metilsulfate is a sympathomimetic drug used for the treatment of low blood pressure. Cardiovascular effects of the new sympathomimetic Amezinium metilsulphate are investigated in 25 patients compared with a control group (n = 25). During spinal/epidural anaesthesia 5 mg amezinium is given i.v. if blood pressure dropped greater than 20 mmHg. from starting-point. A significant recovery of blood pressure (epidural anaesthesia: syst 21%, diast 9%; spinal anaesthesia: syst 13%, diast 6.6%) and a decrease in heart rate (6.8% resp. 4,5%) are thought due to peripheral vasoconstriction. Amezinium proves a stimulating drug for alpha- and beta 1-receptors by stabilising the systemic blood pressure in spinal/epidural anaesthesia. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C12H15N3O5S |
|---|---|
| Molecular Weight | 313.33000 |
| Exact Mass | 313.07300 |
| PSA | 126.83000 |
| LogP | 1.70410 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2933990090 |
|---|
|
~% 
Amezinium methy... CAS#:30578-37-1 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 31, # 9 A p. 1529 - 1533 |
|
~62% 
Amezinium methy... CAS#:30578-37-1 |
| Literature: Reicheneder; Burger; Koenig; Kropp; Lietz; Thyes; Wiersdorff Arzneimittel-Forschung/Drug Research, 1981 , vol. 31, # 9 A p. 1529 - 1533 |
|
~% 
Amezinium methy... CAS#:30578-37-1 |
| Literature: Angewandte Chemie, , vol. 92, # 10 p. 802 - 815 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Supratonin |
| Amezinio metilsulfato [Spanish] |
| Amezinii metilsulfas [INN-Latin] |
| Regulton |
| Ameziniummetilsulfat [German] |
| amexinium methyl sulfate |
| Metilsulfate d'amezinium [INN-French] |
| 4-amino-6-methoxy-1-phenyl-pyridazinium methyl sulfate |
| Amezinium Methyl Sulfate |
| EINECS 250-248-0 |
| Amezinium methylsulfate |
| amezinium metilsulfate |