EO 1428
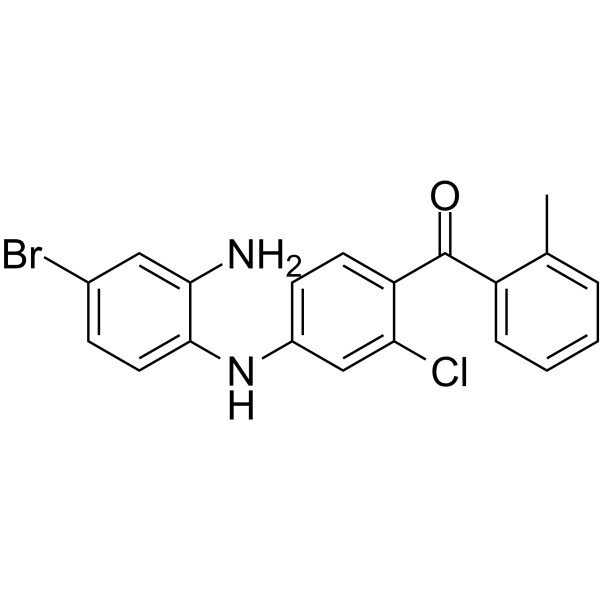
EO 1428 structure
|
Common Name | EO 1428 | ||
|---|---|---|---|---|
| CAS Number | 321351-00-2 | Molecular Weight | 415.71100 | |
| Density | 1.483g/cm3 | Boiling Point | 515.935ºC at 760 mmHg | |
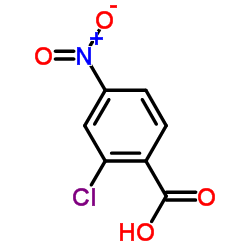
| Molecular Formula | C20H16BrClN2O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 265.828ºC | |
Use of EO 1428EO 1428 is a highly specific inhibitor of p38 of the aminobenzophenone class. EO 1428 (1 μM ) markedly attenuates LPS-induced tumor necrosis factor α-converting enzyme (TACE) activity up-regulation[1]. |
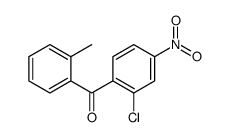
| Name | [4-(2-amino-4-bromoanilino)-2-chlorophenyl]-(2-methylphenyl)methanone |
|---|---|
| Synonym | More Synonyms |
| Description | EO 1428 is a highly specific inhibitor of p38 of the aminobenzophenone class. EO 1428 (1 μM ) markedly attenuates LPS-induced tumor necrosis factor α-converting enzyme (TACE) activity up-regulation[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.483g/cm3 |
|---|---|
| Boiling Point | 515.935ºC at 760 mmHg |
| Molecular Formula | C20H16BrClN2O |
| Molecular Weight | 415.71100 |
| Flash Point | 265.828ºC |
| Exact Mass | 414.01300 |
| PSA | 55.12000 |
| LogP | 6.62190 |
| Index of Refraction | 1.689 |
| HS Code | 2922399090 |
|---|
|
~99% 
EO 1428 CAS#:321351-00-2 |
| Literature: Ottosen, Erik Rytter; Sorensen, Morten Dahl; Bjoerkling, Fredrik; Skak-Nielsen, Tine; Fjording, Marianne Scheel; Aaes, Helle; Binderup, Lise Journal of Medicinal Chemistry, 2003 , vol. 46, # 26 p. 5651 - 5662 |
|
~% 
EO 1428 CAS#:321351-00-2 |
| Literature: Ottosen, Erik Rytter; Sorensen, Morten Dahl; Bjoerkling, Fredrik; Skak-Nielsen, Tine; Fjording, Marianne Scheel; Aaes, Helle; Binderup, Lise Journal of Medicinal Chemistry, 2003 , vol. 46, # 26 p. 5651 - 5662 |
|
~% 
EO 1428 CAS#:321351-00-2 |
| Literature: Ottosen, Erik Rytter; Sorensen, Morten Dahl; Bjoerkling, Fredrik; Skak-Nielsen, Tine; Fjording, Marianne Scheel; Aaes, Helle; Binderup, Lise Journal of Medicinal Chemistry, 2003 , vol. 46, # 26 p. 5651 - 5662 |
|
~% 
EO 1428 CAS#:321351-00-2 |
| Literature: Ottosen, Erik Rytter; Sorensen, Morten Dahl; Bjoerkling, Fredrik; Skak-Nielsen, Tine; Fjording, Marianne Scheel; Aaes, Helle; Binderup, Lise Journal of Medicinal Chemistry, 2003 , vol. 46, # 26 p. 5651 - 5662 |
|
~% 
EO 1428 CAS#:321351-00-2 |
| Literature: Ottosen, Erik Rytter; Sorensen, Morten Dahl; Bjoerkling, Fredrik; Skak-Nielsen, Tine; Fjording, Marianne Scheel; Aaes, Helle; Binderup, Lise Journal of Medicinal Chemistry, 2003 , vol. 46, # 26 p. 5651 - 5662 |
| HS Code | 2922399090 |
|---|---|
| Summary | 2922399090 other amino-aldehydes, amino-ketones and amino-quinones, other than those containing more than one kind of oxygen function; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
| eo 1428 |
| hms3266h20 |
| unii-mv2rhj680o |
| hms3229j13 |
![{4-[(4-bromo-2-nitrophenyl)amino]-2-chlorophenyl}(2-methylphenyl)methanone structure](https://image.chemsrc.com/caspic/266/321378-18-1.png)