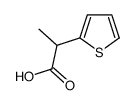
Tiaprofenic Acid

Tiaprofenic Acid structure
|
Common Name | Tiaprofenic Acid | ||
|---|---|---|---|---|
| CAS Number | 33005-95-7 | Molecular Weight | 260.30800 | |
| Density | 1.29 g/cm3 | Boiling Point | 450.3ºC at 760 mmHg | |
| Molecular Formula | C14H12O3S | Melting Point | 96° (isopropyl ether) | |
| MSDS | N/A | Flash Point | 226.1ºC | |
Use of Tiaprofenic AcidTiaprofenic acid is an orally active nonsteroidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic potency. Tiaprofenic acid inhibits prostaglandin synthesis by suppressing cyclo-oxygenase (COX). Tiaprofenic acid can be used in the treatment of rheumatic diseases[1]. |
| Name | Tiaprofenic Acid |
|---|---|
| Synonym | More Synonyms |
| Description | Tiaprofenic acid is an orally active nonsteroidal anti-inflammatory drug (NSAID) with anti-inflammatory and analgesic potency. Tiaprofenic acid inhibits prostaglandin synthesis by suppressing cyclo-oxygenase (COX). Tiaprofenic acid can be used in the treatment of rheumatic diseases[1]. |
|---|---|
| Related Catalog | |
| In Vivo | Female Lewis rats received either Tiaprofenic acid (as an intravenous (iv.) bolus injection of 15 mg/kg and constant infusion of 0.02 mg/min) for 6 h. Tiaprofenic acid results in a significant decrease in serum sulfate concentrations and increase in sulfate clearance ratio. There is also a significant decrease in the fraction of sulfate reabsorbed by the kidneys[2]. |
| References |
| Density | 1.29 g/cm3 |
|---|---|
| Boiling Point | 450.3ºC at 760 mmHg |
| Melting Point | 96° (isopropyl ether) |
| Molecular Formula | C14H12O3S |
| Molecular Weight | 260.30800 |
| Flash Point | 226.1ºC |
| Exact Mass | 260.05100 |
| PSA | 82.61000 |
| LogP | 3.16720 |
| Index of Refraction | 1.612 |
| InChIKey | GUHPRPJDBZHYCJ-UHFFFAOYSA-N |
| SMILES | CC(C(=O)O)c1ccc(C(=O)c2ccccc2)s1 |
| Water Solubility | Practically insoluble in water, freely soluble in acetone, in ethanol (96 per cent) and in methylene chloride. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 2811 6.1/PG III |
|---|---|
| RTECS | XM7580000 |
| Packaging Group | III |
|
~95% 
Tiaprofenic Acid CAS#:33005-95-7 |
| Literature: Zhang, Shuguang; Huang, Shuang; Feng, Chengliang; Cai, Jin; Chen, Junqing; Ji, Min Journal of Chemical Research, 2013 , vol. 37, # 7 p. 406 - 408 |
|
~65% 
Tiaprofenic Acid CAS#:33005-95-7 |
| Literature: Sagami Chemical Research Center Patent: US4242519 A1, 1980 ; |
|
The effect of terpenes on percutaneous absorption of tiaprofenic acid gel.
Arch. Pharm. Res. 33(11) , 1781-8, (2010) Tiaprofenic acid is a potent analgesic and nonsteroidal anti-inflammatory drug (NSAID) and like any other nonsteroidal anti-inflammatory drug, oral administration of the conventional dosage forms of t... |
|
|
Time-resolved spectroscopic study of the photochemistry of tiaprofenic acid in a neutral phosphate buffered aqueous solution from femtoseconds to final products.
J. Phys. Chem. B 117(3) , 811-24, (2013) The photo-decarboxylation and overall reaction mechanism of tiaprofenic acid (TPA) was investigated by femtosecond transient absorption (fs-TA), nanosecond transient absorption (ns-TA), and nanosecond... |
|
|
[New technologies in laparoscopic surgery].
Chirurg. 72(3) , 252-60, (2001) The technology associated with endoscopic surgery continues to evolve as a result of industrial R & D and research within academic surgical departments with interest in surgical endoscopic and remote ... |
| 2-(5-Benzoylthiophen-2-yl)propanoic acid |
| Tiaprofenic acid |
| 2-(5-Benzoylthiophen-2-yl)propionic Acid |

![[5-(1-methoxyethyl)thiophen-2-yl]-phenylmethanone structure](https://image.chemsrc.com/caspic/368/143381-62-8.png) CAS#:143381-62-8
CAS#:143381-62-8 CAS#:6933-26-2
CAS#:6933-26-2 CAS#:5912-44-7
CAS#:5912-44-7![[5-(1-hydroxyethyl)thiophen-2-yl]-phenylmethanone structure](https://image.chemsrc.com/caspic/302/143379-91-3.png) CAS#:143379-91-3
CAS#:143379-91-3