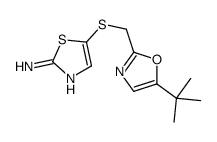
SNS-032 (BMS-387032)

SNS-032 (BMS-387032) structure
|
Common Name | SNS-032 (BMS-387032) | ||
|---|---|---|---|---|
| CAS Number | 345627-80-7 | Molecular Weight | 380.528 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C17H24N4O2S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of SNS-032 (BMS-387032)SNS-032 (BMS-387032) is a selective inhibitor of?CDK2, CDK7, and CDK9 with?IC50s?of 38 nM, 62 nM and 4 nM, respectively. |
| Name | N-[5-[(5-tert-butyl-1,3-oxazol-2-yl)methylsulfanyl]-1,3-thiazol-2-yl]piperidine-4-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | SNS-032 (BMS-387032) is a selective inhibitor of?CDK2, CDK7, and CDK9 with?IC50s?of 38 nM, 62 nM and 4 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
CDK9:4 nM (IC50) CDK2:38 nM (IC50) CDK7:62 nM (IC50) CDK1:480 nM (IC50) CDK4:925 nM (IC50) |
| In Vitro | SNS-032 has low sensitivity to CDK1 and CDK4 with IC50 of 480 nM and 925 nM, respectively. SNS-032 effectively kills chronic lymphocytic leukemia cells in vitro regardless of prognostic indicators and treatment history. Compared with flavopiridol and roscovitine, SNS-032 is more potent, both in inhibition of RNA synthesis and at induction of apoptosis. SNS-032 activity is readily reversible; removal of SNS-032 reactivates RNA polymerase II, which led to resynthesis of Mcl-1 and cell survival[1]. SNS-032 inhibits three dimensional capillary network formations of endothelial cells. SNS-032 completely prevents U87MG cell–mediated capillary formation of HUVECs. In addition, SNS-032 significantly prevents the production of VEGF in both cell lines, SNS-032 prevents in vitro angiogenesis, and this action is attributable to blocking of VEGF. Preclinical studies have shown that SNS-032 induces cell cycle arrest and apoptosis across multiple cell lines[2]. SNS-032 blocks the cell cycle via inhibition of CDKs 2 and 7, and transcription via inhibition of CDKs 7 and 9. SNS-032 activity is unaffected by human serum[3]. SNS-032 induces a dose-dependent increase in annexin V staining and caspase-3 activation. At the molecular level, SNS-032 induces a marked dephosphorylation of serine 2 and 5 of RNA polymerase (RNA Pol) II and inhibits the expression of CDK2 and CDK9 and dephosphorylated CDK7[5]. |
| In Vivo | SNS-032 (15 mg/kg, i.p.) inhibits both xenografted BaF3-T674I cells and KBM5-T315I cells in vivo. SNS-032 abrogates the growth of tumors transplanted in nude mice with downregulation of T674I PDGFRα and T315I-Bcr-Abl[4]. |
| Cell Assay | Cell Titer-Glo (CTG) luminescent assay is performed to measure the growth curves of both HUVECs and U87MG cells. U87MG cells and HUVECs (2×103 cells/well) are seeded in a 96-well microplate in a final volume of 100 mL. After 24 hours, cells are treated with various doses of SNS-032 (0-0.5 mM) for 24, 48, or 72 hours. After completion of the treatment, 100 mL of CTG solution is added to each well and incubated for 20 minutes at room temperature in the dark. Lysate (50 mL) is transferred to a 96-well white plate, and luminescence is measured by POLARstar OPTIMA. Percent cell growth is calculated by considering 100% growth at the time of SNS-032 addition. |
| Animal Admin | Nude nu/nu BALB/c mice are housed in barrier facilities with a 12-hour light-dark cycle, with food and water available ad libitum. A mixture of 1×107 of BaF3-T674I cells with Matrigel or KBM5-T315I cells (3×107) are inoculated subcutaneously on the flanks of 4- to 6-week-old male nude mice. Tumors are measured every other day with use of calipers. Tumor volumes are calculated by the following formula: a2×b×0.4, where a is the smallest diameter and b is the diameter perpendicular to a. Four days after subcutaneous inoculation, when tumors are palpable (appr 100 mm3), mice are randomized to receive treatment with vehicle (tissue culture medium containing DMSO 0.1% v/v) or SNS-032 (15 mg/kg injected intraperitoneally every 2 days) for about 2 weeks. SNS-032 is dissolved in tissue culture grade DMSO before dilution. The body weight, feeding behavior, and motor activity of each animal are monitored as indicators of general health. The animals are then euthanized, and tumor xenografts are immediately removed, weighed, stored, and fixed. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C17H24N4O2S2 |
| Molecular Weight | 380.528 |
| Exact Mass | 380.134064 |
| PSA | 133.59000 |
| LogP | 2.79 |
| Index of Refraction | 1.607 |
| InChIKey | OUSFTKFNBAZUKL-UHFFFAOYSA-N |
| SMILES | CC(C)(C)c1cnc(CSc2cnc(NC(=O)C3CCNCC3)s2)o1 |
| Storage condition | -20℃ |
|
~79% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: CURIS, INC. Patent: WO2009/36016 A1, 2009 ; Location in patent: Page/Page column 55 ; |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: Journal of Medicinal Chemistry, , vol. 47, # 7 p. 1719 - 1728 |
|
~% 
SNS-032 (BMS-387032) CAS#:345627-80-7 |
| Literature: WO2009/36016 A1, ; |
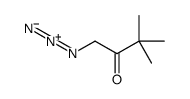
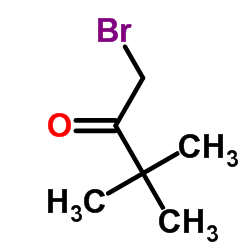
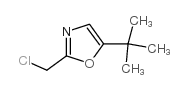
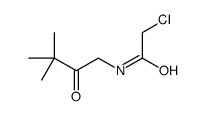
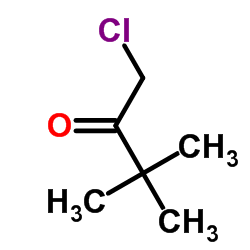
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| N-[5-({[5-(2-Methyl-2-propanyl)-1,3-oxazol-2-yl]methyl}sulfanyl)-1,3-thiazol-2-yl]-4-piperidinecarboxamide |
| piperidine-4-carboxylic acid [5-(5-tert-butyl-oxazol-2-ylmethylsulfanyl)-thiazol-2-yl]-amide |
| 4-Piperidinecarboxamide,N-(5-(((5-(1,1-dimethylethyl)-2-oxazolyl)methyl)thio)-2-thiazolyl) |
| 1-diMethylethyl)-2-oxazolyl]Methyl]thio]-2-thiazolyl] |
| N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide |
| 4-Piperidinecarboxamide, N-[5-[[[5-(1,1-dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]- |
| N-(5-((5-Tert-Butyloxazol-2-yl)methylthio)thiazol-2-yl)piperidine-4-carboxamide |
| N-(5-(((5-(1,1-dimethylethyl)-2-oxazolyl)methyl)thio)-2-thiazolyl)-4-piperidinecarboxamide |
| N-(5-{[(5-tert-Butyl-1,3-oxazol-2-yl)methyl]sulfanyl}-1,3-thiazol-2-yl)piperidine-4-carboxamide |
| SNS-032 |
| BMS-387032 |
| BMS-3870032 |