L-Thioproline
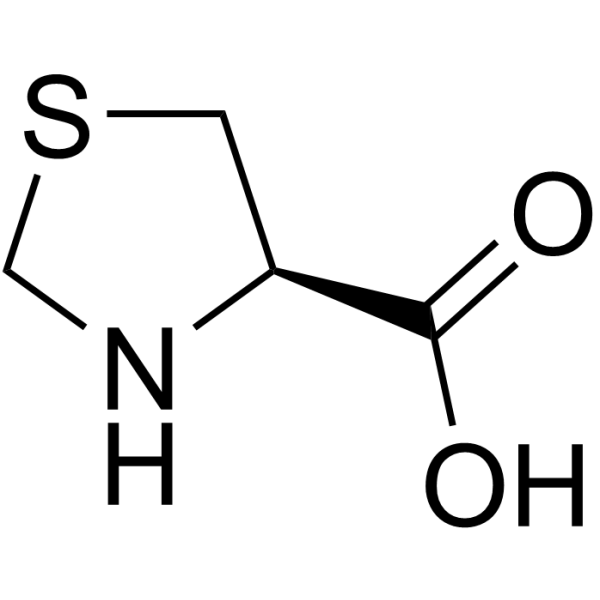
L-Thioproline structure
|
Common Name | L-Thioproline | ||
|---|---|---|---|---|
| CAS Number | 34592-47-7 | Molecular Weight | 133.169 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 350.3±37.0 °C at 760 mmHg | |
| Molecular Formula | C4H7NO2S | Melting Point | 190-200 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 165.7±26.5 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of L-Thioproline(R)-Thiazolidine-4-carboxylic acid is a proline derivative[1]. |
| Name | L-Thioproline |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-Thiazolidine-4-carboxylic acid is a proline derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 350.3±37.0 °C at 760 mmHg |
| Melting Point | 190-200 °C (dec.)(lit.) |
| Molecular Formula | C4H7NO2S |
| Molecular Weight | 133.169 |
| Flash Point | 165.7±26.5 °C |
| Exact Mass | 133.019745 |
| PSA | 74.63000 |
| LogP | -0.39 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.566 |
| InChIKey | DZLNHFMRPBPULJ-VKHMYHEASA-N |
| SMILES | O=C(O)C1CSCN1 |
| Water Solubility | 28.5 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H312-H315-H319-H332-H335 |
| Precautionary Statements | P261-P280-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36/37/39-S36 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | XJ5425500 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29341000 |
|
~98% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Kaupp, Gerd; Schmeyers, Jens; Boy, Juergen Tetrahedron, 2000 , vol. 56, # 36 p. 6899 - 6911 |
|
~93% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Refouvelet; Pellegrini; Robert; Crini; Blacque; Kubicki Journal of Heterocyclic Chemistry, 2000 , vol. 37, # 6 p. 1425 - 1430 |
|
~% 
L-Thioproline CAS#:34592-47-7 |
| Literature: US6605608 B1, ; US 6605608 B1 |
|
~% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Journal of Biological Chemistry, , vol. 114, p. 348 Journal of Biological Chemistry, , vol. 121, p. 542,545 |
|
~78% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Shiraiwa, Tadashi; Kataoka, Kazuo; Kurokawa, Hidemoto Chemistry Letters, 1987 , p. 2041 - 2042 |
|
~% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Journal of Organic Chemistry, , vol. 74, # 11 p. 4289 - 4297 |
|
~% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Tetrahedron, , vol. 51, # 10 p. 3015 - 3024 |
|
~% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 65, # 1 p. 267 - 273 |
|
~% 
L-Thioproline CAS#:34592-47-7 |
| Literature: Bulletin of the Chemical Society of Japan, , vol. 60, # 9 p. 3277 - 3284 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Three-dimensional quantitative structure-activity relationship analyses of substrates of the human proton-coupled amino acid transporter 1 (hPAT1).
Bioorg. Med. Chem. 19 , 6409-18, (2011) The proton-coupled amino acid transporter hPAT1 has recently gained much interest due to its ability to transport small drugs thereby allowing their oral administration. A three-dimensional quantitati... |
|
|
Design, synthesis and biological evaluation of 2-(substituted phenyl)thiazolidine-4-carboxylic acid derivatives as novel tyrosinase inhibitors.
Biochimie 94(2) , 533-40, (2012) Herein we describe the design, synthesis and biological activities of 2-(substituted phenyl)thiazolidine-4-carboxylic acid derivatives as novel tyrosinase inhibitors. The target compounds 2a-2j were d... |
|
|
Successful computer guided planned synthesis of (4R)-thiazolidine carboxylic acid and its 2-substituted analogues as urease inhibitors.
Mol. Divers. 10(2) , 223-31, (2006) By using internal combinatorial library we were able to identify (4R)-thiazolidines carboxylic acid and its 2-substituted analogs as active inhibitors of urease. Molecular modeling and virtual screeni... |
| H-THZ-OH |
| L-Thiazolidine-4-car |
| (R)-4-Thiazolidinecarboxylic acid |
| EINECS 252-106-3 |
| L-THC |
| THIAPROLINE |
| (4R)-4-Thiazolidinecarboxylic acid |
| (4R)-thiazolidine-4-carboxylic acid |
| 1,3-Thiazolidine-2-carboxylic acid |
| Thiazolidinecarboxylic acid (VAN) |
| THIAZOLIDINECARBOXYLIC ACID |
| 2-Thiazolidinecarboxylic acid |
| DL-Thiazolidinecarboxylic acid |
| L(-)-Thiazolidine-4-carboxylicacid |
| L(-)-Thiazolidine-4-Carboxylic Acid |
| L-Thiazolidine-4-carboxylic acid |
| H-L-THZ-OH |
| MFCD00005212 |
| (4R)-1,3-Thiazolidine-4-carboxylic acid |
| L-4-Thiazolidinecarboxylic acid |
| L-Thioproline |
| thiazolidinecarboxylicacid |
| (R)-(-)-4-Thiazolidinecarboxylic acid |
| L- thiazolidine-4-carboxylic acid |
| THIOPROLINE |
| H-THIOPRO-OH |
| L-THIAPROLINE |
| (R)-Thiazolidine-4-carboxylic acid |
| (R)-(-)-Thiazolidine-4-carboxylic Acid |
| L-thiazoline-4-carboxylic acid |
| 4-Thiazolidinecarboxylic acid, (4R)- |


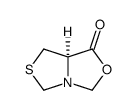
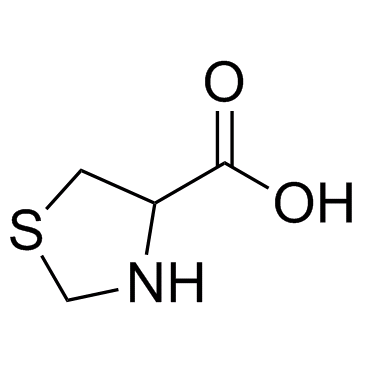
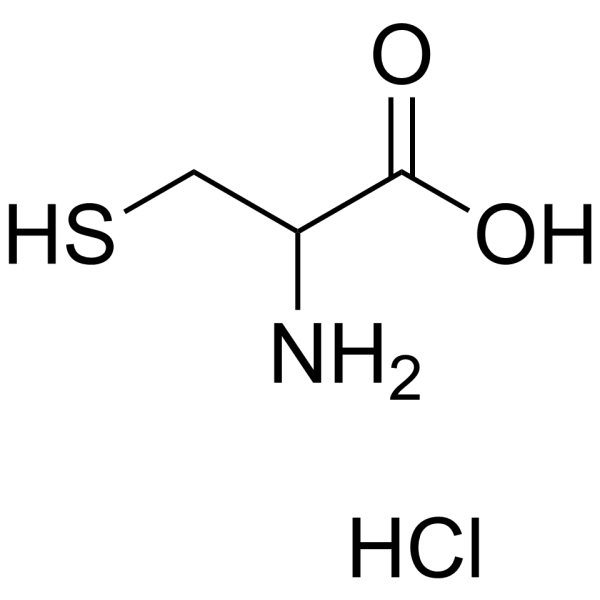
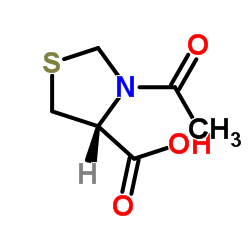 CAS#:54323-50-1
CAS#:54323-50-1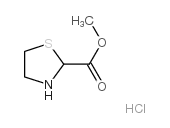 CAS#:50703-06-5
CAS#:50703-06-5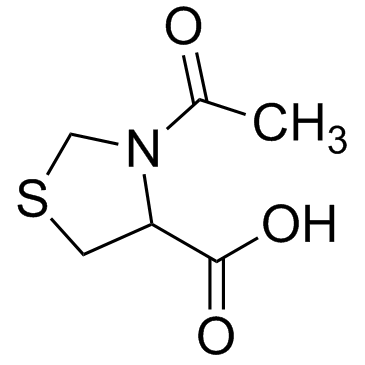 CAS#:5025-82-1
CAS#:5025-82-1 CAS#:121808-62-6
CAS#:121808-62-6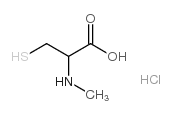 CAS#:14344-46-8
CAS#:14344-46-8 CAS#:122946-43-4
CAS#:122946-43-4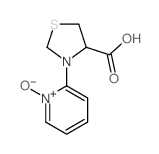 CAS#:70665-28-0
CAS#:70665-28-0
