Ziyuglycoside II
Modify Date: 2025-08-25 19:03:28
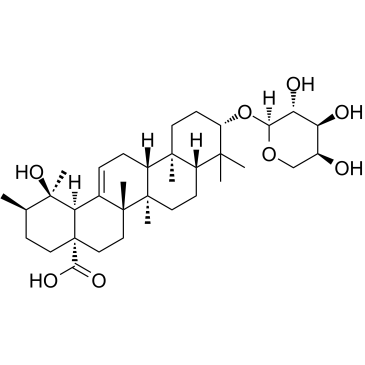
Ziyuglycoside II structure
|
Common Name | Ziyuglycoside II | ||
|---|---|---|---|---|
| CAS Number | 35286-59-0 | Molecular Weight | 604.814 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 716.2±60.0 °C at 760 mmHg | |
| Molecular Formula | C35H56O8 | Melting Point | 260-263℃ | |
| MSDS | N/A | Flash Point | 218.6±26.4 °C | |
Use of Ziyuglycoside IIZiyuglycoside II is a triterpenoid saponin compound extracted from Sanguisorba officinalis L.. Ziyuglycoside II induces reactive oxygen species (ROS) production and apoptosis. Anti-inflammation and anti-cancer effect[1]. |
| Name | (3β)-3-(α-L-Arabinopyranosyloxy)-19-hydroxyurs-12-en-28-oic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Ziyuglycoside II is a triterpenoid saponin compound extracted from Sanguisorba officinalis L.. Ziyuglycoside II induces reactive oxygen species (ROS) production and apoptosis. Anti-inflammation and anti-cancer effect[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Ziyuglycoside II (10-60 μM; 24 h and 48 h) inhibits MDA-MB-435 cells growth in a dose-dependent manner. The IC50 of Ziyuglycoside II at 24 h and 48 h is 5.92 μM and 4.74 μM, respectively[1]. Ziyuglycoside II (5-25 μM) induces G0/G1 and S phase arrest in MDA-MB-435 cells at 24 h[1]. Ziyuglycoside II (5-25 μM; 24 hours) significantly increases apoptotic rate of MDA-MB-435 cells[1]. Ziyuglycoside II (5-25 μM; 24 hours) increases expressions of both p53 and p21 in MDA-MB-435 cells, which effect is dose-dependent[1]. Cell Viability Assay[1] Cell Line: MDA-MB-435 cells Concentration: 10, 20, 30, 40, 50, 60 μM Incubation Time: 24 hours and 48 hours Result: The IC50 at 24 h and 48 h was 5.92 μM and 4.74 μM, respectively. Cell Cycle Analysis[1] Cell Line: MDA-MB-435 cells Concentration: 5, 10, 25 μM Incubation Time: 24 hours Result: Induced G0/G1 and S phase arrest. Apoptosis Analysis[1] Cell Line: MDA-MB-435 cells Concentration: 5, 10, 25 μM Incubation Time: 24 hours Result: The apoptotic rate was significantly increased in comparison to that of the control. Western Blot Analysis[1] Cell Line: MDA-MB-435 cells Concentration: 5, 10, 25 μM Incubation Time: 24 hours Result: Treatment resulted in increased expressions of both p53 and p21. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 716.2±60.0 °C at 760 mmHg |
| Melting Point | 260-263℃ |
| Molecular Formula | C35H56O8 |
| Molecular Weight | 604.814 |
| Flash Point | 218.6±26.4 °C |
| Exact Mass | 604.397522 |
| PSA | 136.68000 |
| LogP | 7.68 |
| Vapour Pressure | 0.0±5.2 mmHg at 25°C |
| Index of Refraction | 1.588 |
| Storage condition | 2-8°C |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| Urs-12-en-28-oic acid, 3-(α-L-arabinopyranosyloxy)-19-hydroxy-, (3β)- |
| zirconyl carbonate |
| (3β)-3-(α-L-Arabinopyranosyloxy)-19-hydroxyurs-12-en-28-oic acid |
| Ziyuglycoside II |
| Zirconium carbonate oxide |
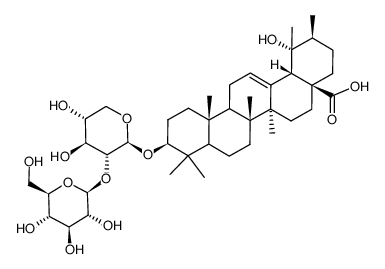 CAS#:83725-19-3
CAS#:83725-19-3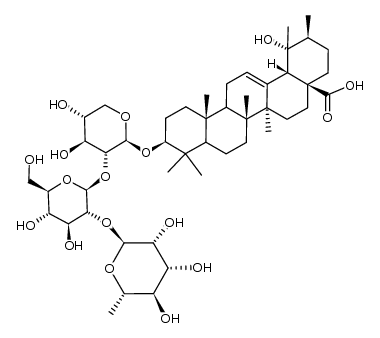 CAS#:108906-69-0
CAS#:108906-69-0
