2-hydroxyestrone
Modify Date: 2025-08-25 10:54:27
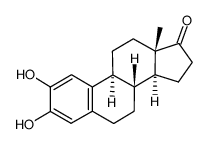
2-hydroxyestrone structure
|
Common Name | 2-hydroxyestrone | ||
|---|---|---|---|---|
| CAS Number | 362-06-1 | Molecular Weight | 286.36500 | |
| Density | 1.241g/cm3 | Boiling Point | 481.3ºC at 760 mmHg | |
| Molecular Formula | C18H22O3 | Melting Point | 199-201ºC | |
| MSDS | N/A | Flash Point | 259ºC | |
Use of 2-hydroxyestrone2-Hydroxyestrone (Catecholestrone) is a specific receptor-mediated antiestrogenic agent. 2-Hydroxyestrone is anticarcinogenic[1][2]. |
| Name | 2-hydroxyestrone |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Hydroxyestrone (Catecholestrone) is a specific receptor-mediated antiestrogenic agent. 2-Hydroxyestrone is anticarcinogenic[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite Estrogen receptor |
| In Vitro | 2-Hydroxyestrone exhibits antiestrogen action on MCF-7 human breast cancer cells. Addition of 2-Hydroxyestrone to the cell cultures in concentration of 1-1000 nM has no effect on cell growth and proliferation because of rapid O-methylation of the catechol estrogen by catechol O-methyltransferase which is highly active in these cells. In the presence of quinalizarin, a potent catechol O-methyltransferase inhibitor which reduces the O-methylation of the steroid, 10 and 100 nM 2-Hydroxyestrone markedly suppresses the growth and proliferation of the cells[2]. Cell Proliferation Assay[2] Cell Line: Human breast cancer cell lines MCF-7, MDA-MB-231, and MDA-MB-330 Concentration: 1-1000 nM Incubation Time: 6 days Result: 10 and 100 nM markedly suppressed the growth and proliferation of the cells in the presence of quinalizarin. The tumor cell growth-inhibitory action of the catechol estrogen was neutralized by the presence of 1 nM estradiol. |
| In Vivo | Levels of both 2-Hydroxyestrone (2-OHE1; 2 mg/kg; administered ip.) and 2-Hydroxyestrone 4-N-acetylcysteine thioether (2-OHE1 4SR) in rats treated with 2-Hydroxyestrone were significantly different between the induced and noninduced groups[3]. Animal Model: Female and male Sprague-Dawley rats (6 weeks old)[3] Dosage: 2 mg/kg Administration: Administered i.p. Result: Levels of both 2-OHE1 and 2-OHE1 4SR in rats treated with 2-OHE1 were significantly different between the induced and noninduced groups. |
| References |
[1]. H L Bradlow, et al. 2-Hydroxyestrone: the 'good' estrogen. J Endocrinol. 1996 Sep;150 Suppl:S259-65. |
| Density | 1.241g/cm3 |
|---|---|
| Boiling Point | 481.3ºC at 760 mmHg |
| Melting Point | 199-201ºC |
| Molecular Formula | C18H22O3 |
| Molecular Weight | 286.36500 |
| Flash Point | 259ºC |
| Exact Mass | 286.15700 |
| PSA | 57.53000 |
| LogP | 3.52300 |
| Index of Refraction | 1.609 |
| InChIKey | SWINWPBPEKHUOD-JPVZDGGYSA-N |
| SMILES | CC12CCC3c4cc(O)c(O)cc4CCC3C1CCC2=O |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|
| Precursor 8 | |
|---|---|
| DownStream 3 | |
| 3-Methoxypicolinimidamidehydrochloride |
| 3-O-Methyl 17|A-Estradiol |
| 2-hydroxyestron |
| 2-Hydroxyestrone |
 CAS#:53-16-7
CAS#:53-16-7 CAS#:53875-00-6
CAS#:53875-00-6 CAS#:14984-43-1
CAS#:14984-43-1![(8S,9S,13S,14S)-3-hydroxy-13-methyl-2-nitro-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one Structure](https://image.chemsrc.com/caspic/229/5976-73-8.png) CAS#:5976-73-8
CAS#:5976-73-8 CAS#:734-32-7
CAS#:734-32-7 CAS#:362-08-3
CAS#:362-08-3 CAS#:23463-05-0
CAS#:23463-05-0 CAS#:5976-64-7
CAS#:5976-64-7 CAS#:5976-63-6
CAS#:5976-63-6