CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XZ4250000
-
CHEMICAL NAME :
-
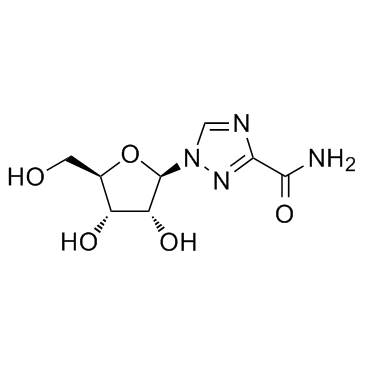
1,2,4-Triazole-3-carboxamide, 1-beta-D-ribofuranosyl-
-
CAS REGISTRY NUMBER :
-
36791-04-5
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
32
-
MOLECULAR FORMULA :
-
C8-H12-N4-O5
-
MOLECULAR WEIGHT :
-
244.24
-
WISWESSER LINE NOTATION :
-
T5OTJ B1Q CQ DQ E- AT5NN DNJ CVZ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2700 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - active as anti-cancer agent
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
4 gm/kg
-
TOXIC EFFECTS :
-
Tumorigenic - active as anti-cancer agent
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1500 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Kidney, Ureter, Bladder - other changes Blood - hemorrhage
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
1 gm/kg/10D-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Blood - changes in bone marrow (not otherwise specified) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
220 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Blood - changes in platelet count Nutritional and Gross Metabolic - changes in calcium
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
220 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Blood - changes in platelet count Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
440 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Blood - changes in bone marrow (not otherwise specified) Blood - changes in platelet count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
37500 ug/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3150 mg/kg
-
SEX/DURATION :
-
male 90 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
9 gm/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3750 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2500 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2500 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2500 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
Micronucleus test
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Cytogenetic analysis
MUTATION DATA
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TEST SYSTEM :
-
Rodent - rabbit Kidney
-
DOSE/DURATION :
-
4900 ug/L
-
REFERENCE :
-
BCPCA6 Biochemical Pharmacology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.1- 1958- Volume(issue)/page/year: 38,1771,1989 *** REVIEWS *** TOXICOLOGY REVIEW JACTDZ Journal of the American College of Toxicology. (Mary Ann Liebert, Inc., 1651 Third Ave., New York, NY 10128) V.1-12, 1982-1993. Discontinued. Volume(issue)/page/year: 9(5),551,1990
|


![[(2R,3R,4R,5R)-3,4-diacetyloxy-5-(3-cyano-1,2,4-triazol-1-yl)oxolan-2-yl]methyl acetate Structure](https://image.chemsrc.com/caspic/248/40371-99-1.png) CAS#:40371-99-1
CAS#:40371-99-1 CAS#:57198-04-6
CAS#:57198-04-6 CAS#:38934-69-9
CAS#:38934-69-9 CAS#:3641-08-5
CAS#:3641-08-5 CAS#:118-00-3
CAS#:118-00-3 CAS#:72933-99-4
CAS#:72933-99-4 CAS#:33763-06-3
CAS#:33763-06-3 CAS#:58-96-8
CAS#:58-96-8 CAS#:114283-60-2
CAS#:114283-60-2 CAS#:114283-62-4
CAS#:114283-62-4 CAS#:120362-26-7
CAS#:120362-26-7 CAS#:120362-25-6
CAS#:120362-25-6 CAS#:120615-22-7
CAS#:120615-22-7![[(2R,3R,4R,5R)-5-(3-carbamoyl-1,2,4-triazol-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxyphosphonic acid structure](https://image.chemsrc.com/caspic/170/40925-28-8.png) CAS#:40925-28-8
CAS#:40925-28-8 CAS#:52663-90-8
CAS#:52663-90-8
