Ifosfamide
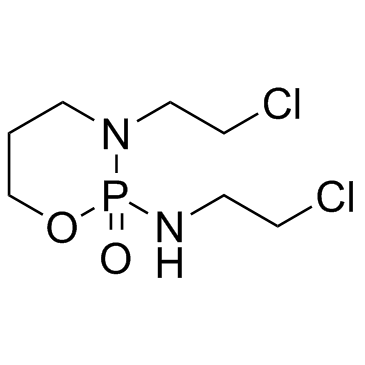
Ifosfamide structure
|
Common Name | Ifosfamide | ||
|---|---|---|---|---|
| CAS Number | 3778-73-2 | Molecular Weight | 261.086 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 336.1±52.0 °C at 760 mmHg | |
| Molecular Formula | C7H15Cl2N2O2P | Melting Point | 48ºC | |
| MSDS | Chinese USA | Flash Point | 157.1±30.7 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of IfosfamideIfosfamide is an alkylating chemotherapeutic agent with activity against a wide range of tumors. |
| Name | ifosfamide |
|---|---|
| Synonym | More Synonyms |
| Description | Ifosfamide is an alkylating chemotherapeutic agent with activity against a wide range of tumors. |
|---|---|
| Related Catalog | |
| Target |
DNA Alkylator[1] |
| In Vitro | Ifosfamide is an alkylating chemotherapeutic agent with activity against a wide range of tumors[1]. Ifosfamide is activated by the cytochrome P450 family. Ifosfamide (0-5 mM) suppresses the viability of CYP2B1-expressing C8III-1 cells, while it is cytotoxic to the non-CYP2B1-expressing CrFK cells only at high concentration (5 mM)[2]. CYP BM3 mutants activates Ifosfamide, and Ifosfamide shows inhibitory activity against human U2OS cells[3]. |
| In Vivo | Ifosfamide (50 mg/kg, i.p.) treatment before mating increases percentage of post-implantation loss and resorbs fetuses in pregnant rats. Ifosfamide also (50 mg/kg, i.p.) decreases the progesterone in the serum of pregnant rats. However, Ifosfamide causes no obvious difference with the control rats at 25 mg/kg. Ifosfamide (25, 50 mg/kg, i.p.) induces apoptosis and histological changes in the placentas and prenatal rats, most sensitive fetal organs are the brain, liver and kidney[1]. |
| Cell Assay | In a 3-cm dish, 4 × 104 cells are seeded in 2 mL of medium. The next day, Ifosfamide is added to final concentrations from 0 to 5 mM. After six additional days the medium is removed, the cells washed with PBS, and either stained or counted[2]. |
| Animal Admin | Rats[1] Prior to mating, female rats are divided into four groups of eight: group 1, untreated negative controls; group 2, rats injected i.p. with 1 mL 0.9% NaCl; group 3, rats injected i.p. with 25 mg/kg Ifosfamide; group 4, rats injected i.p. with 50 mg/kg Ifosfamide. After injection of Ifosfamide once daily for 5 consecutive days, three females are placed in a cage with one untreated male for up to 1 week. Vaginal smears are examined daily to determine pregnancy. The first 24 h period following mating is designated day one of pregnancy if sperm are detected. The pregnant females are housed singly and observed daily for signs of toxicity and abortion. All pregnant animals are sacrificed by decapitation on day 18 of gestation. Cardiac blood (2.5 -3 mL/rat) is collected in nonheparinized test tubes, centrifuged at 3,000× g for 30 min and the serum is decanted and stored at -70°C until used for hormone assay. After blood collection, uteri and both ovaries are removed, washed in saline solution and the corpora lutea of pregnancy are counted visually, and the number of implantation sites, resorption sites and viable fetuses are counted in each uterine horn. Each fetus is removed from its umbilical cord, weighed and the crown rump (CR) length is measured. Fetuses are examined for external malformation and the placental weights are recorded. Fetuses and placentas of control and treated groups are fixed in 10% neutral buffered formalin for immunohistochemical and histological examination[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 336.1±52.0 °C at 760 mmHg |
| Melting Point | 48ºC |
| Molecular Formula | C7H15Cl2N2O2P |
| Molecular Weight | 261.086 |
| Flash Point | 157.1±30.7 °C |
| Exact Mass | 260.024811 |
| PSA | 51.38000 |
| LogP | 0.23 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.506 |
| InChIKey | HOMGKSMUEGBAAB-UHFFFAOYSA-N |
| SMILES | O=P1(NCCCl)OCCCN1CCCl |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H319 |
| Precautionary Statements | Missing Phrase - N15.00950417-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | 26-45 |
| RIDADR | UN 3249 |
| RTECS | RP6050000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2934999090 |
| Precursor 10 | |
|---|---|
| DownStream 6 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Comparative study of the effects of antituberculosis drugs and antiretroviral drugs on cytochrome P450 3A4 and P-glycoprotein.
Antimicrob. Agents Chemother. 58(6) , 3168-76, (2014) Predicting drug-drug interactions (DDIs) related to cytochrome P450 (CYP), such as CYP3A4 and one of the major drug transporters, P-glycoprotein (P-gp), is crucial in the development of future chemoth... |
|
|
Disulfiram modulates stemness and metabolism of brain tumor initiating cells in atypical teratoid/rhabdoid tumors.
Neuro. Oncol. 17 , 810-21, (2015) Atypical teratoid/rhabdoid tumors (AT/RT) are among the most malignant pediatric brain tumors. Cells from brain tumors with high aldehyde dehydrogenase (ALDH) activity have a number of characteristics... |
|
|
Non conventional biological treatment based on Trametes versicolor for the elimination of recalcitrant anticancer drugs in hospital wastewater.
Chemosphere 136 , 9-19, (2015) This work presents a study about the elimination of anticancer drugs, a group of pollutants considered recalcitrant during conventional activated sludge wastewater treatment, using a biological treatm... |
| P-(2-AMINOPROPYL)PHENOL |
| 1-p-Hydroxyphenyl-2-propylamine |
| 2H-1,3,2-Oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide |
| (+/-)-Hydroxyamphetamine |
| rac-iphosphamide |
| N,3-bis(2-chloroéthyl)-1,3,2-oxazaphosphinan-2-amine-2-oxyde |
| MFCD00057374 |
| ISOPHOSPHAMIDE |
| Ifex (N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine-2-oxide |
| 2-Amino-1-(p-hydroxyphenyl)propane |
| 4-(2-aminopropyl)phenol |
| Norpholedrine |
| Ifosfamide |
| 3-(2-chloroethyl)-2-[(2-chloroethyl)-amino]-2-oxo-1,3,2-oxazaphosphorinane |
| Holoxan |
| ilfosfamide |
| 1-(4-hydroxyphenyl)-2-amino-propane |
| iphosphamide |
| IFEX |
| N,3-Bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide |
| p-Hydroxyamphetamine |
| Dl-p-(2-aminopropyl)phenol |
| (+)-4-hydroxyamphetamine |
| Hydroxyamphetamin |
| ifosphamide |
| 3-(2-Chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide |
| Mitoxana |
| EINECS 223-237-3 |
| l-1-(4-hydroxyphenyl)-2-aminopropane |
| 2-(4-Hydroxyphenyl)-1-methylethylamine |
| Ifex N,3-Bis(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine-2-oxide |
 CAS#:29102-47-4
CAS#:29102-47-4![(R)-2-aziridin-1-yl-3-((R)-1-phenyl-ethyl)-[1,3,2]oxazaphosphinane 2-oxide Structure](https://image.chemsrc.com/caspic/473/72578-61-1.png) CAS#:72578-61-1
CAS#:72578-61-1![(2-chloro-ethyl)-[(S)-2-oxo-3-((R)-1-phenyl-ethyl)-2λ5-[1,3,2]oxazaphosphinan-2-yl]-amine Structure](https://image.chemsrc.com/caspic/207/72578-68-8.png) CAS#:72578-68-8
CAS#:72578-68-8![(2-chloro-ethyl)-[(R)-2-oxo-3-((R)-1-phenyl-ethyl)-2λ5-[1,3,2]oxazaphosphinan-2-yl]-amine Structure](https://image.chemsrc.com/caspic/245/72578-67-7.png) CAS#:72578-67-7
CAS#:72578-67-7 CAS#:81485-04-3
CAS#:81485-04-3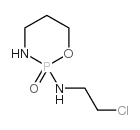 CAS#:36761-83-8
CAS#:36761-83-8![3-chloroacetyl-2-(2-chloro-ethylamino)-[1,3,2]oxazaphosphinane (S)-2-oxide Structure](https://image.chemsrc.com/caspic/228/72578-72-4.png) CAS#:72578-72-4
CAS#:72578-72-4![3-(2-Chloroactyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine-2-oxide Structure](https://image.chemsrc.com/caspic/266/72578-71-3.png) CAS#:72578-71-3
CAS#:72578-71-3 CAS#:31190-87-1
CAS#:31190-87-1![(1'R,2S)-2-[(1'-methylbenzyl)amino]-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide Structure](https://image.chemsrc.com/caspic/142/84681-43-6.png) CAS#:84681-43-6
CAS#:84681-43-6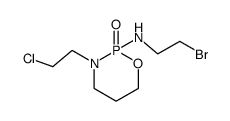 CAS#:104149-14-6
CAS#:104149-14-6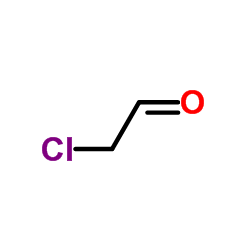 CAS#:107-20-0
CAS#:107-20-0 CAS#:107-02-8
CAS#:107-02-8 CAS#:42436-20-4
CAS#:42436-20-4 CAS#:39800-28-7
CAS#:39800-28-7
