Triptolide
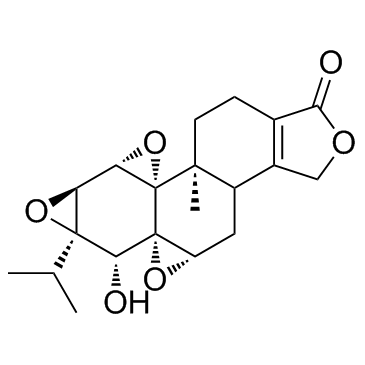
Triptolide structure
|
Common Name | Triptolide | ||
|---|---|---|---|---|
| CAS Number | 38748-32-2 | Molecular Weight | 360.401 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 601.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H24O6 | Melting Point | 226-227°C | |
| MSDS | USA | Flash Point | 220.7±25.0 °C | |
Use of TriptolideTriptolide is a diterpenoid triepoxide extracted from the root of Tripterygium wilfordii with immunosuppressive, anti-inflammatory and antiproliferative effects. Triptolide is a NF-κB activation inhibitor. |
| Name | triptolide |
|---|---|
| Synonym | More Synonyms |
| Description | Triptolide is a diterpenoid triepoxide extracted from the root of Tripterygium wilfordii with immunosuppressive, anti-inflammatory and antiproliferative effects. Triptolide is a NF-κB activation inhibitor. |
|---|---|
| Related Catalog | |
| Target |
HSP90 MDM-2/p53:47-73 nM (IC50) |
| In Vitro | Triptolide induces apoptosis in cultured and primary Chronic Lymphocytic Leukemia (CLL) B-cells. Treatment of CD19+ B cells with Triptolide, induces a dose-dependent increase in apoptosis in cultured and primary CLL cells. Triptolide is selectively toxic to both high risk (n=5) and low risk CLL (n=12) B cells (10 to 50 nM range) while largely sparing normal B-cells (n=5). Consistent with the inhibition of heat-shock induced HSP transcription, treatment with Triptolide attenuates heat-shock induced expression of HSPs[1]. Triptolide is a natural product derived from the Chinese plant Tripterygium wilfordii, is reported to exhibit antitumor effects in a broad range of cancers. Triptolide inhibits MDM2 expression in a dose-dependent manner, even at low concentrations spanning 20-100 nM in acute lymphoblastic leukemia (ALL) cells. Triptolide exhibits strongly cytotoxic activity in all 8 cell lines having native MDM2 overexpression, with IC50 values range from 47 to 73 nM. Triptolide exhibits much less cytotoxic effect on EU-4 cells that express very low level of MDM2, while it effectively kill these cells when MDM2 is stably transfected (IC50 values: 725 nM vs. 88 nM)[2]. Differentiated PC12 cells are incubated with different concentrations of Triptolide (0.01, 0.1, and 1 nM) in the presence of 10 μM Aβ25-35 for 24 hours and MTT assay is used to detect the effect of Triptolide. The results show that Aβ25-35 can decrease the cell viability and when treated with Triptolide the viability of differentiated PC12 cells is significantly increased. The results indicate that Triptolide can alleviate cellular damage caused by Aβ25-35, which means that Triptolide has a neuroprotective effect[3]. |
| In Vivo | The Triptolide (TP) plasma concentrations are declined rapidly in mice after receive an intravenous dose. After 2h of injection, the Triptolide concentrations are dropped below the lower limit of quantification for all three groups. A comparison of the parameters is made between the control and the treated groups to assess the effect of P-gp inhibition on the Triptolide exposure and elimination. Treatment with the mdr1a-siRNA can significantly enhance the Triptolide plasma exposure, with the Cmax increases from 413±74 to 510±94 ng/mL (P<0.05) and the AUC from 103.5±9.6 to 154.3±30.2 ng•h/mL (P<0.05). In the concomitant group with Tariquidar, the significantly increased AUC is also noted, from 103.5±9.6 of the control to 145.9±24.6 ng•h/mL of the Triptolide+Tariquidar group (P<0.05). Accordingly, the total body clearance of Triptolide in mice is remarkably decreased, from 9564±1024.2 mL/min/kg of the control to 6576.4±1438.5 (P<0.05) and 5755.4±1200.1 mL/min/kg (P<0.05) for Triptolide+Tariquidar and Triptolide+mdr1a-siRNA groups, respectively[4]. |
| Cell Assay | The viability of differentiated PC12 cells treated with different concentrations of Triptolide. After differentiated PC12 cells are cultured on 96-well plates with RPMI 1640 medium for stabilization, differentiated PC12 cells are incubated with different concentrations of Triptolide (0.01, 0.1, and 1 nM) for 24 hours. The concentrations in this study are chosen. Then cell viability is determined by the MTT assay. Each condition and experiment is repeated three times[3]. |
| Animal Admin | Mice[4] Male BALB/C mice (weight, 18-22 g) are used. For Triptolide (TP) plasma kinetic study and toxicological evaluation, mice are divided into four groups (n=5 each) to collect blood and tissue samples: (1) normal+saline group; (2) 1.0 mg/kg Triptolide+15 nmol negative control (NC) siRNA-siRNA group; (3) 1.0 mg/kg Triptolide+15 nmol mdr1a-siRNA group; (4) 1.0 mg/kg Triptolide+10 mg/kg Tariquidar group. In order to avoid the complication caused by drug absorption or possible intestinal first-pass effect, Triptolide and the inhibitor are intravenously administrated to mice. The siRNA group is intravenously injected with NC-siRNA or mdr1a-siRNA 2 days before Triptolide dose. For Triptolide+Tariquidar group, the mice are received an intravenous Tariquidar dose 20 min prior to the Triptolide injection. Blood samples are collected at 2, 5, 10, 15, 30, 60 and 120 min after Triptolide dosing. To assess the liver exposure of Triptolide, liver tissue samples are collected from another set of mice at 5, 30, 60 and 120 min after dosing. Three Triptolide groups are design for this experiment, including Triptolide+NC-siRNA group, Triptolide+mdr1a-siRNA group and Triptolide+Tariquidar group. The liver tissue samples are weighed and then homogenized in 10 volume (w:v) of ice-cold saline. The concentrations of Triptolide in plasma and liver tissue are measured by a validated LC-MS/MS method. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 601.7±55.0 °C at 760 mmHg |
| Melting Point | 226-227°C |
| Molecular Formula | C20H24O6 |
| Molecular Weight | 360.401 |
| Flash Point | 220.7±25.0 °C |
| Exact Mass | 360.157288 |
| PSA | 84.12000 |
| LogP | 1.27 |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.647 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~31% 
Triptolide CAS#:38748-32-2 |
| Literature: Aoyagi, Yutaka; Hitotsuyanagi, Yukio; Hasuda, Tomoyo; Fukaya, Haruhiko; Takeya, Koichi; Aiyama, Ritsuo; Matsuzaki, Takeshi; Hashimoto, Shusuke Bioorganic and Medicinal Chemistry Letters, 2011 , vol. 21, # 10 p. 3046 - 3049 |
|
~49% 
Triptolide CAS#:38748-32-2 |
| Literature: Yang, Dan; Ye, Xiang-Yang; Xu, Ming Journal of Organic Chemistry, 2000 , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
Triptolide CAS#:38748-32-2 |
| Literature: Yang, Dan; Ye, Xiang-Yang; Xu, Ming Journal of Organic Chemistry, 2000 , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
Triptolide CAS#:38748-32-2 |
| Literature: Yang, Dan; Ye, Xiang-Yang; Xu, Ming Journal of Organic Chemistry, 2000 , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
Triptolide CAS#:38748-32-2 |
| Literature: Yang, Dan; Ye, Xiang-Yang; Xu, Ming Journal of Organic Chemistry, 2000 , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
Triptolide CAS#:38748-32-2 |
| Literature: Yang, Dan; Ye, Xiang-Yang; Xu, Ming Journal of Organic Chemistry, 2000 , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
Triptolide CAS#:38748-32-2 |
| Literature: Yang, Dan; Ye, Xiang-Yang; Xu, Ming Journal of Organic Chemistry, 2000 , vol. 65, # 7 p. 2208 - 2217 |
|
~28% 
Triptolide CAS#:38748-32-2
Detail
|
| Literature: Aoyagi, Yutaka; Hitotsuyanagi, Yukio; Hasuda, Tomoyo; Fukaya, Haruhiko; Takeya, Koichi; Aiyama, Ritsuo; Matsuzaki, Takeshi; Hashimoto, Shusuke Bioorganic and Medicinal Chemistry Letters, 2011 , vol. 21, # 10 p. 3046 - 3049 |
|
Biological activity and safety of Tripterygium extract prepared by sodium carbonate extraction.
Molecules 17(9) , 11113-23, (2012) The commercial preparation named “Tripterygium glycosides” prepared by column chromatography has been used for the treatment of inflammatory and autoimmune diseases with significant efficacy but concu... |
|
|
Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation.
Nat. Commun. 6 , 7815, (2015) Chromosome condensation is a hallmark of mitosis in eukaryotes and is a prerequisite for faithful segregation of genetic material to daughter cells. Here we show that condensin, which is essential for... |
|
|
Cardiosphere-derived cells from pediatric end-stage heart failure patients have enhanced functional activity due to the heat shock response regulating the secretome.
Stem Cells 33(4) , 1213-29, (2015) We have demonstrated that human neonatal cardiosphere-derived cells (CDCs) derived from the young are more regenerative due to their robust secretome. However, it is unclear how the decompensated pedi... |
| MFCD00210565 |
| (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-6-Hydroxy-6a-isopropyl-8b-methyl-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydrotrisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one |
| (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)trisoxireno[4b,5:6,7:8a,9]phenanthro[1,2-c]furan-1(3H)-one |
| triptolid |
| Triptolide (PG490) |
| EINECS 300-006-3 |
| (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-6-hydroxy-8b-methyl-6a-(propan-2-yl)-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydrotrisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one |
| (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)-3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-6a-isopropyl-8b-methyltrisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one |
| TRIPTOLIDE TLC |
| Triptolide |
| Trisoxireno[6,7:8a,9:4b,5]phenanthro[1,2-c]furan-1(3H)-one, 3b,4,4a,6,6a,7a,7b,8b,9,10-decahydro-6-hydroxy-8b-methyl-6a-(1-methylethyl)-, (3bS,4aS,5aS,6R,6aR,7aS,7bS,8aS,8bS)- |
| Triptolide(PG490) |
| ,7as,7bs,8as,8bs) |
| PG490 |
| triptergium wilfordii |

![(5aS,9aR,10aS,11aR)-4,5,5a,10a,11,11a-Hexahydro-5a-methyl-8-(1-methylethyl)-1H-oxireno[8a,9]phenanthro[1,2-c]furan-3,9-dione structure](https://www.chemsrc.com/extcaspic/251/225787-03-1.png)
![(3bR,9bS)-7-isopropyl-6-methoxy-9b-methyl-3b,4,10,11-tetrahydrophenanthro[1,2-c]furan-1,5(3H,9bH)-dione structure](https://image.chemsrc.com/caspic/224/225786-93-6.png)

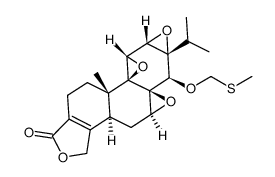 CAS#:847440-49-7
CAS#:847440-49-7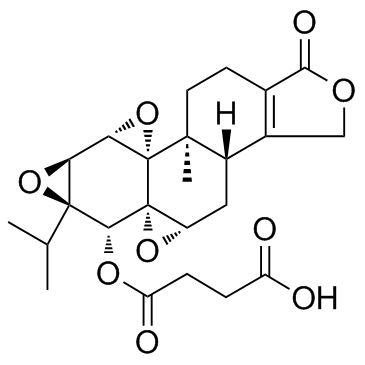 CAS#:195883-06-8
CAS#:195883-06-8
