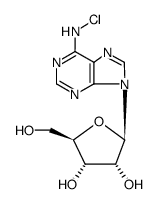
N6-Cyclopentyladenosine
N6-Cyclopentyladenosine structure
|
Common Name | N6-Cyclopentyladenosine | ||
|---|---|---|---|---|
| CAS Number | 41552-82-3 | Molecular Weight | 335.36 | |
| Density | 1.78g/cm3 | Boiling Point | 673.4ºC at 760 mmHg | |
| Molecular Formula | C15H21N5O4 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 361.1ºC | |
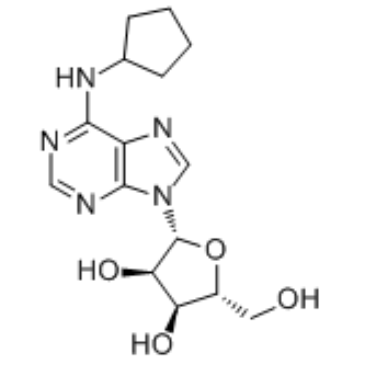
Use of N6-CyclopentyladenosineN6-Cyclopentyladenosine (CPA) is a selective Adenosine A1 receptor agonist, with Ki values of 2.3 nM, 790 nM and 43 nM for human A1, A2A and A3 receptors, respectively[1][2]. |
| Name | (2R,3R,4S,5R)-2-(6-(Cyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol |
|---|---|
| Synonym | More Synonyms |
| Description | N6-Cyclopentyladenosine (CPA) is a selective Adenosine A1 receptor agonist, with Ki values of 2.3 nM, 790 nM and 43 nM for human A1, A2A and A3 receptors, respectively[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 2.3 nM (A1), 790 nM (A2A), 43 nM(A3)[1]. |
| References |
| Density | 1.78g/cm3 |
|---|---|
| Boiling Point | 673.4ºC at 760 mmHg |
| Molecular Formula | C15H21N5O4 |
| Molecular Weight | 335.36 |
| Flash Point | 361.1ºC |
| Exact Mass | 335.15900 |
| PSA | 125.55000 |
| Index of Refraction | 1.816 |
| Storage condition | 2-8℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~79% 
N6-Cyclopentyla... CAS#:41552-82-3 |
| Literature: Ottria, Roberta; Casati, Silvana; Baldoli, Erika; Maier, Jeanette A.M.; Ciuffreda, Pierangela Bioorganic and Medicinal Chemistry, 2010 , vol. 18, # 23 p. 8396 - 8402 |
|
~70% 
N6-Cyclopentyla... CAS#:41552-82-3 |
| Literature: Vittori, Sauro; Lorenzen, Anna; Stannek, Christina; Costanzi, Stefano; Volpini, Rosaria; Ijzerman, Adriaan P.; Von Frijtag Drabbe Kunzel, Jakobien K.; Cristalli, Gloria Journal of Medicinal Chemistry, 2000 , vol. 43, # 2 p. 250 - 260 |
|
~85% 
N6-Cyclopentyla... CAS#:41552-82-3 |
| Literature: Fletcher, Steven Tetrahedron Letters, 2010 , vol. 51, # 22 p. 2948 - 2950 |
|
~% 
N6-Cyclopentyla... CAS#:41552-82-3 |
| Literature: Vittori, Sauro; Lorenzen, Anna; Stannek, Christina; Costanzi, Stefano; Volpini, Rosaria; Ijzerman, Adriaan P.; Von Frijtag Drabbe Kunzel, Jakobien K.; Cristalli, Gloria Journal of Medicinal Chemistry, 2000 , vol. 43, # 2 p. 250 - 260 |
|
~% 
N6-Cyclopentyla... CAS#:41552-82-3 |
| Literature: Vittori, Sauro; Lorenzen, Anna; Stannek, Christina; Costanzi, Stefano; Volpini, Rosaria; Ijzerman, Adriaan P.; Von Frijtag Drabbe Kunzel, Jakobien K.; Cristalli, Gloria Journal of Medicinal Chemistry, 2000 , vol. 43, # 2 p. 250 - 260 |
|
~% 
N6-Cyclopentyla... CAS#:41552-82-3 |
| Literature: Inotek Pharmaceuticals Corporation Patent: US2007/238694 A1, 2007 ; Location in patent: Page/Page column 130 ; |
|
The effect of pH and ion channel modulators on human placental arteries.
PLoS ONE 9(12) , e114405, (2014) Chorionic plate arteries (CPA) are located at the maternofetal interface where they are able to respond to local metabolic changes. Unlike many other types of vasculature, the placenta lacks nervous c... |
|
|
Acid-sensing ion channel 3 decreases phosphorylation of extracellular signal-regulated kinases and induces synoviocyte cell death by increasing intracellular calcium.
Arthritis. Res. Ther. 16(3) , R121, (2014) Acid-sensing ion channel 3 (ASIC3) is expressed in synoviocytes, activated by decreases in pH, and reduces inflammation in animal models of inflammatory arthritis. The purpose of the current study was... |
|
|
Chemoenzymatic Syntheses of Sialylated Oligosaccharides Containing C5-Modified Neuraminic Acids for Dual Inhibition of Hemagglutinins and Neuraminidases.
Chemistry 21 , 10903-12, (2015) A fast chemoenzymatic synthesis of sialylated oligosaccharides containing C5-modified neuraminic acids is reported. Analogues of GM3 and GM2 ganglioside saccharidic portions where the acetyl group of ... |
| N6-Cyclopentyladenosine |


![9-[2,3,5-TRI-O-ACETYL-BETA-D-RIBOFURANOSYL]-2,6-DICHLOROPURINE structure](https://image.chemsrc.com/caspic/019/3056-18-6.png)