fenbendazole
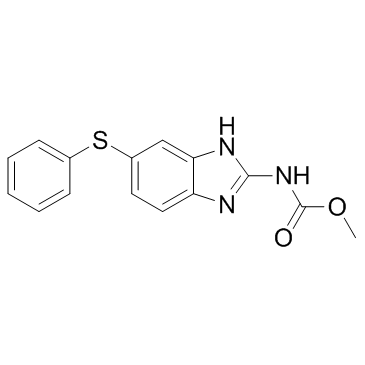
fenbendazole structure
|
Common Name | fenbendazole | ||
|---|---|---|---|---|
| CAS Number | 43210-67-9 | Molecular Weight | 299.348 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 541.4±42.0 °C at 760 mmHg | |
| Molecular Formula | C15H13N3O2S | Melting Point | 233°C | |
| MSDS | Chinese USA | Flash Point | 281.2±27.9 °C | |
Use of fenbendazoleFenbendazole is a broad spectrum benzimidazole anthelmintic used against gastrointestinal parasites.Target: AntiparasiticFenbendazole is a broad spectrum benzimidazole anthelmintic used against gastrointestinal parasites including: giardia, roundworms, hookworms, whipworms, the taenia species of tapeworms(It is effective against the Taenia species of tapeworm but not against the common tapeworm, Dipylidium caninum.), pinworms, aelurostrongylus, paragonimiasis, strongyles and strongyloides and can be administered to sheep, cattle, horses, fish, dogs, cats, rabbits and seals. Drug interactions may occur if using bromsalan flukicides such as dibromsalan and tribromsalan. Abortions in cattle and death in sheep have been reported after using these medications together. Fenbendazole is poorly absorbed from the gastrointestinal tract in most species. The LD50 in laboratory animals exceeds 10 g/kg when administered orally. From Wikipedia. |
| Name | fenbendazole |
|---|---|
| Synonym | More Synonyms |
| Description | Fenbendazole is a broad spectrum benzimidazole anthelmintic used against gastrointestinal parasites.Target: AntiparasiticFenbendazole is a broad spectrum benzimidazole anthelmintic used against gastrointestinal parasites including: giardia, roundworms, hookworms, whipworms, the taenia species of tapeworms(It is effective against the Taenia species of tapeworm but not against the common tapeworm, Dipylidium caninum.), pinworms, aelurostrongylus, paragonimiasis, strongyles and strongyloides and can be administered to sheep, cattle, horses, fish, dogs, cats, rabbits and seals. Drug interactions may occur if using bromsalan flukicides such as dibromsalan and tribromsalan. Abortions in cattle and death in sheep have been reported after using these medications together. Fenbendazole is poorly absorbed from the gastrointestinal tract in most species. The LD50 in laboratory animals exceeds 10 g/kg when administered orally. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 541.4±42.0 °C at 760 mmHg |
| Melting Point | 233°C |
| Molecular Formula | C15H13N3O2S |
| Molecular Weight | 299.348 |
| Flash Point | 281.2±27.9 °C |
| Exact Mass | 299.072845 |
| PSA | 92.31000 |
| LogP | 4.34 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.679 |
| InChIKey | HDDSHPAODJUKPD-UHFFFAOYSA-N |
| SMILES | COC(=O)Nc1nc2ccc(Sc3ccccc3)cc2[nH]1 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~89% 
fenbendazole CAS#:43210-67-9 |
| Literature: Cortes, Eduardo Cortes; Mendoza, Rafael Sosa; Gutierrez, Maximiliano Santibanez; De Cortes, Olivia Garcia-Mellado Journal of Heterocyclic Chemistry, 2004 , vol. 41, # 2 p. 273 - 276 |
|
~% 
fenbendazole CAS#:43210-67-9 |
| Literature: Cortes, Eduardo Cortes; Mendoza, Rafael Sosa; Gutierrez, Maximiliano Santibanez; De Cortes, Olivia Garcia-Mellado Journal of Heterocyclic Chemistry, 2004 , vol. 41, # 2 p. 273 - 276 |
|
~% 
fenbendazole CAS#:43210-67-9 |
| Literature: Cortes, Eduardo Cortes; Mendoza, Rafael Sosa; Gutierrez, Maximiliano Santibanez; De Cortes, Olivia Garcia-Mellado Journal of Heterocyclic Chemistry, 2004 , vol. 41, # 2 p. 273 - 276 |
|
~% 
fenbendazole CAS#:43210-67-9 |
| Literature: Cortes, Eduardo Cortes; Mendoza, Rafael Sosa; Gutierrez, Maximiliano Santibanez; De Cortes, Olivia Garcia-Mellado Journal of Heterocyclic Chemistry, 2004 , vol. 41, # 2 p. 273 - 276 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Development and validation of an ultra high performance liquid chromatography tandem mass spectrometry method for simultaneous determination of sulfonamides, quinolones and benzimidazoles in bovine milk.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 962 , 20-9, (2014) A simple, sensitive and reliable analytical method was developed for the simultaneous determination of 38 veterinary drugs (18 sulfonamides, 11 quinolones and 9 benzimidazoles) and 8 metabolites of be... |
|
|
Epidemiology. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales.
Science 347(6218) , 175-7, (2015) Parasitic worms modulate host immune responses in ways that affect microbial co-infections. For this reason, anthelmintic therapy may be a potent tool for indirectly controlling microbial pathogens. H... |
|
|
Enantiomeric behaviour of albendazole and fenbendazole sulfoxides in domestic animals: pharmacological implications.
Vet. J. 181(3) , 241-50, (2009) Albendazole and fenbendazole are methylcarbamate benzimidazole anthelmintics extensively used to control gastrointestinal parasites in domestic animals. These parent compounds are metabolised to alben... |
| Fenbendazole |
| HOE-881V |
| Methyl [5-(phenylsulfanyl)-1H-benzimidazol-2-yl]carbamate |
| Methyl hydrogen [6-(phenylsulfanyl)-1H-benzimidazol-2-yl]carbonimidate |
| SAFEGARD |
| MFCD00144301 |
| 5-(Phenylthio)-2-benzimidazolecarbamic Acid Methyl Ester |
| hoe881 |
| fenbendazol |
| Carbamic acid, N-[6-(phenylthio)-1H-benzimidazol-2-yl]-, methyl ester |
| EINECS 256-145-7 |
| Febendazole |
| Fenbedazole |
| [5-(Phenylthio)benzimidazol-2-yl]carbamic Acid Methyl Ester |
| Methanol, 1-methoxy-1-[[6-(phenylthio)-1H-benzimidazol-2-yl]imino]-, (E)- |
| PANACUR |
| Fenbion |
| Methyl 5-(phenylthio)-2-benzimidazolecarbamate |
| Methyl [5-(Phenylthio)benzimidazol-2-yl]carbamate |
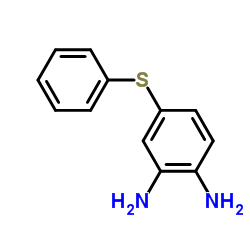
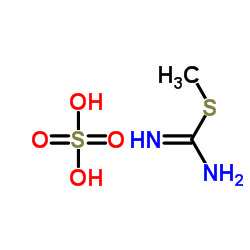
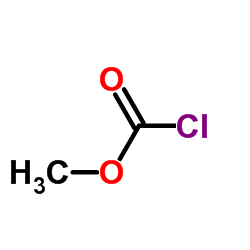
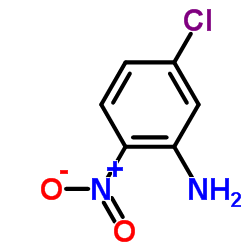


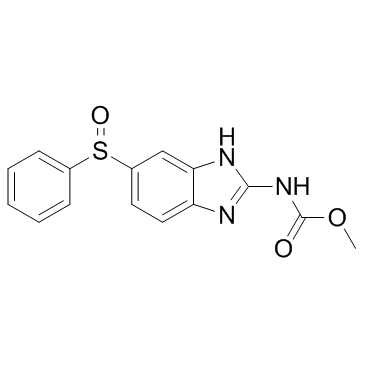 CAS#:53716-50-0
CAS#:53716-50-0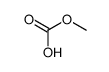 CAS#:7456-87-3
CAS#:7456-87-3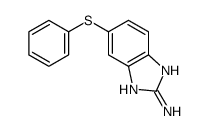 CAS#:53065-28-4
CAS#:53065-28-4 CAS#:59530-20-0
CAS#:59530-20-0
