Cyclopamine
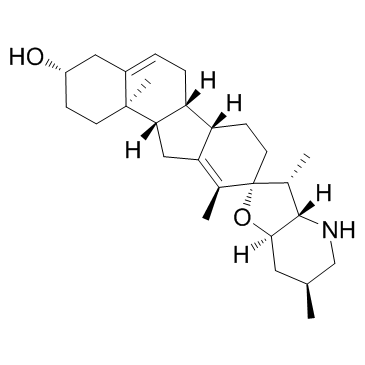
Cyclopamine structure
|
Common Name | Cyclopamine | ||
|---|---|---|---|---|
| CAS Number | 4449-51-8 | Molecular Weight | 411.620 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 550.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H41NO2 | Melting Point | 236-238ºC | |
| MSDS | N/A | Flash Point | 286.9±30.1 °C | |
Use of CyclopamineCyclopamine is a Hedgehog (Hh) pathway antagonist with an IC50 of 46 nM in the Hh cell assay. |
| Name | Cyclopamine |
|---|---|
| Synonym | More Synonyms |
| Description | Cyclopamine is a Hedgehog (Hh) pathway antagonist with an IC50 of 46 nM in the Hh cell assay. |
|---|---|
| Related Catalog | |
| Target |
IC50: 46 nM (Hedgehog, in Hh cell assay)[1] |
| In Vitro | Treatment with small molecule Hh inhibitors such as HhAntag and the natural product Cyclopamine, both binding to Smo, induces tumor remission in a genetic mouse model of medulloblastoma[1]. Cyclopamine is a Hedgehog (Hh) pathway antagonist. Cyclopamine suppresses cell growth. Cyclopamine (3 μM) suppression of Hh pathway activity and growth in digestive tract tumour cell lines correlates with expression of PTCHmRNA[2]. Cyclopamine is a steroidal alkaloid that inhibits Hh signalling through direct interaction with Smo[3]. |
| In Vivo | Cyclopamine causes durable regression of xenograft tumors. Tumors in Cyclopamine-treated animals, regress completely by 12 days[2]. Cyclopamine (1.2 mg) treatment blocks tumour formation of human pancreatic adenocarcinoma cells after transplantation into nude mice[3]. |
| Cell Assay | Cells are cultured in triplicate in 96-well plates in assay media to which 5E1 monoclonal antibody, ShhNp and/or Cyclopamine (3 μM) are added at 0 h at concentrations indicated in the main text. Viable cell mass is determined by optical density measurements at 490 nm (OD490) at 2 and 4 days using the CellTiter96 colorimetric assay. Relative growth is calculated as OD (day 4)-OD (day 2)/OD (day 2)[2]. |
| Animal Admin | Mice[3] A total of 0.1 mL Hanks’ balanced salt solution and matrigel (1:1) containing 2×106 cells is injected subcutaneously into CD-1 nude mice. Tumors are grown for 4 days to a minimum volume of 125 mm3; treatment is initiated simultaneously for all subjects. Mice are injected subcutaneously with vector alone (triolein:ethanol 4:1 v/v) or a Cyclopamine suspension (1.2 mg per mouse in triolein:ethanol 4:1 v/v) daily for 7 days. At the end of the treatment period, tumours are excised from mice, weighed and then fixed for 3 h at 4°C with 4% paraformaldehyde, embedded in paraffin wax and sectioned (6 µm). Apoptotic cells are identified by TUNEL using recombinant Tdt. Sections are then counterstained with eosin. Eight ×20-magnified fields from regions corresponding to the exterior, middle and interior of two control and two cyclopamine-treated tumours are chosen at random. We counted the number of TUNEL-positive nuclei manually. Haematoxylin/eosin staining is done. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 550.8±50.0 °C at 760 mmHg |
| Melting Point | 236-238ºC |
| Molecular Formula | C27H41NO2 |
| Molecular Weight | 411.620 |
| Flash Point | 286.9±30.1 °C |
| Exact Mass | 411.313721 |
| PSA | 41.49000 |
| LogP | 5.44 |
| Vapour Pressure | 0.0±3.4 mmHg at 25°C |
| Index of Refraction | 1.583 |
| InChIKey | QASFUMOKHFSJGL-LAFRSMQTSA-N |
| SMILES | CC1=C2CC3C(CC=C4CC(O)CCC43C)C2CCC12OC1CC(C)CNC1C2C |
| Storage condition | 2-8°C |
| Stability | Store in Freezer at - 20°C |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi: Irritant; |
|---|---|
| Safety Phrases | 22-24/25 |
| WGK Germany | 3 |
| RTECS | GY0750000 |
|
~91% 
Cyclopamine CAS#:4449-51-8 |
| Literature: Giannis, Athanassios; Heretsch, Philipp; Sarli, Vasiliki; Stoessel, Anne Angewandte Chemie - International Edition, 2009 , vol. 48, # 42 p. 7911 - 7914 |
|
~51% 
Cyclopamine CAS#:4449-51-8 |
| Literature: Suginome, Hiroshi; Yonekura, Norihisa; Masamune, Tadashi Bulletin of the Chemical Society of Japan, 1980 , vol. 53, # 1 p. 210 - 213 |
|
~% 
Cyclopamine CAS#:4449-51-8 |
| Literature: Hamon, Florian; Renoux, Brigitte; Chadeneau, Corinne; Muller, Jean-Marc; Papot, Sebastien European Journal of Medicinal Chemistry, 2010 , vol. 45, # 4 p. 1678 - 1682 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| 11-DEOXYJERVINE |
| alkaloidv |
| Spiro[9H-benzo[a]fluorene-9,2'(3'H)-furo[3,2-b]pyridin]-3-ol, 1,2,3,3'a,4,4',5',6,6',6a,6b,7,7',7'a,8,11,11a,11b-octadecahydro-3',6',10,11b-tetramethyl-, (3S,3'R,3a'S,6'S,6aS,6bS,7a'R,9R,11aS,11bR)- |
| deoxojervine |
| 11-Desoxo-jervin |
| Veratraman-3-ol, 17,23-epoxy-, (3β,23β)- |
| Cyclopamine |
| (3β,22S,23R)-17,23-Epoxyveratraman-3-ol |
| Spiro(9H-benzo(a)fluorene-9,2'(3'H)-furo(3,2-b)pyridin)-3-ol, 1,2,3,3'a,4,4',5',6,6',6a,6b,7,7',7'a,8,11,11a,11b-octadecahydro-3',6',10,11b-tetramethyl-, (2'R,3S,3'R,3'aS,6'S,6aS,6bS,7'aR,11aS,11bR)- |
| 11-deoxojervine |
| MFCD01735266 |
| (3β,17β,22S,23R)-17,23-Epoxyveratraman-3-ol |
| 11-deoxo-jervin |
![(2'R,3S,3'R,3a'S,6aS,6bS,6'S,7a'R,11aS,11bR)-3',6',10,11b-tetramethyl-4'-(phenylsulfonyl)-1,2,3,3a',4,4',5',6,6a,6b,6',7,7',7a',8,11,11a,11b-octadecahydro-3'H-spiro[benzo[a]fluorene-9,2'-furo[3,2-b]pyridin]-3-ol structure](https://image.chemsrc.com/caspic/086/1198184-95-0.png)



![(3'R,3'aS,6'S,6aS,6bS,7'aR,9R,11aS,11bR)-3',6',10,11b-tetramethylspiro[1,2,5,6,6a,6b,7,8,11,11a-decahydrobenzo[a]fluorene-9,2'-3a,4,5,6,7,7a-hexahydro-3H-furo[3,2-b]pyridine]-3-one structure](https://image.chemsrc.com/caspic/436/14410-98-1.png) CAS#:14410-98-1
CAS#:14410-98-1
