Ajmalicine
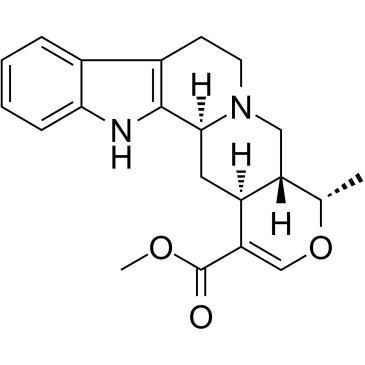
Ajmalicine structure
|
Common Name | Ajmalicine | ||
|---|---|---|---|---|
| CAS Number | 483-04-5 | Molecular Weight | 352.427 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 524.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H24N2O3 | Melting Point | 258°C (rough estimate) | |
| MSDS | Chinese USA | Flash Point | 270.7±30.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AjmalicineAjmalicine (Raubasine) is found in herbs of Catharanthus roseus, is an antihypertensive drug used in the treatment of high blood pressure, decreases peripheral resistance and blood pressure[1].Ajmalicine (Raubasine) is an adrenolytic drug which preferentially blocks alpha 1-adrenoceptor than alpha 2-adrenoceptor[2].Ajmalicine (Raubasine) is an reversible non-competitive nicotine receptor antagonist with an IC50 of 72.3 μM[3].Ajmalicine (Raubasine) acts preferentially at postsynaptic sites, competitively antagonizes the effect of noradrenaline on postsynaptic alpha-adrenoceptor with a pA2 value of 6.57, blocks the inhibitory effect of clonidine with an pA2 value of 6.2[4]. |
| Name | Raubasine |
|---|---|
| Synonym | More Synonyms |
| Description | Ajmalicine (Raubasine) is found in herbs of Catharanthus roseus, is an antihypertensive drug used in the treatment of high blood pressure, decreases peripheral resistance and blood pressure[1].Ajmalicine (Raubasine) is an adrenolytic drug which preferentially blocks alpha 1-adrenoceptor than alpha 2-adrenoceptor[2].Ajmalicine (Raubasine) is an reversible non-competitive nicotine receptor antagonist with an IC50 of 72.3 μM[3].Ajmalicine (Raubasine) acts preferentially at postsynaptic sites, competitively antagonizes the effect of noradrenaline on postsynaptic alpha-adrenoceptor with a pA2 value of 6.57, blocks the inhibitory effect of clonidine with an pA2 value of 6.2[4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 524.0±50.0 °C at 760 mmHg |
| Melting Point | 258°C (rough estimate) |
| Molecular Formula | C21H24N2O3 |
| Molecular Weight | 352.427 |
| Flash Point | 270.7±30.1 °C |
| Exact Mass | 352.178680 |
| PSA | 54.56000 |
| LogP | 2.88 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.656 |
| InChIKey | GRTOGORTSDXSFK-XJTZBENFSA-N |
| SMILES | COC(=O)C1=COC(C)C2CN3CCc4c([nH]c5ccccc45)C3CC12 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 4 | |
|---|---|
| DownStream 0 | |
|
Determination of terpenoid indole alkaloids in hairy roots of Rhazya stricta (Apocynaceae) by GC-MS.
Phytochem. Anal. 26 , 331-8, (2015) Rhazya stricta Decne. (Apocynaceae) is a medicinal plant rich in terpenoid indole alkaloids (TIAs), some of which possess important pharmacological properties. The study material including transgenic ... |
|
|
Synergistic and cytotoxic action of indole alkaloids produced from elicited cell cultures of Catharanthus roseus.
Pharm. Biol. 51(3) , 304-10, (2013) Catharanthus roseus (L.) G. Don (Apocynaceae) is a medicinal plant that produces more than 130 alkaloids, with special attention given to the production of the anti-hypertensive monomeric indole alkal... |
|
|
A differential response to chemical elicitors in Catharanthus roseus in vitro cultures.
Biotechnol. Lett. 31(4) , 591-5, (2009) The effects of methyl jasmonate, salicylic acid and ethylene on alkaloid accumulation in in vitro cell suspension, hairy roots and rootless shoot cultures of Catharanthus roseus were analyzed. Ajmalic... |
| Circolene |
| EINECS 207-589-5 |
| Pytetrahydroserpentine |
| Vincein |
| Ranitol |
| yochimbine |
| Vinceine |
| Methyl (4S,4aR,13bS,14aS)-4-methyl-4a,5,7,8,13,13b,14,14a-octahydro-4H-indolo[2,3-a]pyrano[3,4-g]quinolizine-1-carboxylate |
| Lamuran |
| Methyl-(4S,4aR,13bS,14aS)-4-methyl-4a,5,7,8,13,13b,14,14a-octahydro-4H-indolo[2,3-a]pyrano[3,4-g]chinolizin-1-carboxylat |
| Methyl-(19α)-19-methyl-16,17-didehydro-18-oxayohimban-16-carboxylat |
| fluoroajmalicine |
| Einecs 224-471-9 |
| d-Yohimbine |
| δ-Yohimbine |
| RAUBASINE HYDROCHLORIDE |
| AJMALICINE HCL |
| (19α)-16,17-Didehydro-19-methyl-oxayohimban-16-carboxylic acid methyl ester |
| Methyl (19α)-19-methyl-16,17-didehydro-18-oxayohimban-16-carboxylate |
| Raubasil |
| Raubasine |
| Ajmalicine |
| RAUBASIN |
| ajmalicine,monohydrochloride |
| Oxayohimban-16-carboxylic acid, 16,17-didehydro-19-methyl-, methyl ester, (19α)- |
| Tensyl |
| Sarpan |
| AJMALICIN HCL |
| Tetrahydroserpentine |
| Rauvasan |
| Vincain |
 CAS#:67-56-1
CAS#:67-56-1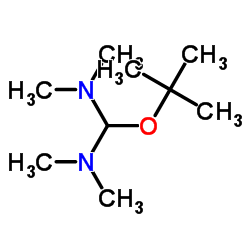 CAS#:5815-08-7
CAS#:5815-08-7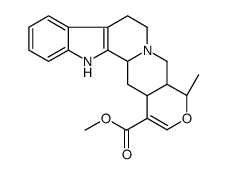 CAS#:483-03-4
CAS#:483-03-4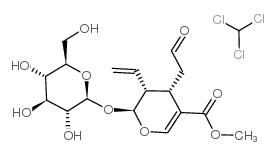 CAS#:19351-63-4
CAS#:19351-63-4
