Kynurenic acid

Kynurenic acid structure
|
Common Name | Kynurenic acid | ||
|---|---|---|---|---|
| CAS Number | 492-27-3 | Molecular Weight | 189.167 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 358.4±42.0 °C at 760 mmHg | |
| Molecular Formula | C10H7NO3 | Melting Point | 275 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 170.5±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Kynurenic acidKynurenic acid, an endogenous tryptophan metabolite, is a broad-spectrum antagonist targeting NMDA, glutamate, α7 nicotinic acetylcholine receptor. Kynurenic acid is also a selective ligand of the GPR35 receptor. |
| Name | kynurenic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Kynurenic acid, an endogenous tryptophan metabolite, is a broad-spectrum antagonist targeting NMDA, glutamate, α7 nicotinic acetylcholine receptor. Kynurenic acid is also a selective ligand of the GPR35 receptor. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | GPR35 functions as a receptor for the kynurenine pathway intermediate kynurenic acid. Kynurenic acid elicits calcium mobilization and inositol phosphate production in a GPR35-dependent manner in the presence of G qi/o chimeric G proteins. Kynurenic acid stimulates [35S]guanosine 5′-O-(3-thiotriphosphate) binding in GPR35-expressing cells, an effect abolished by pertussis toxin treatment. Kynurenic acid also induces the internalization of GPR35[1]. KYNA’s neuroinhibitory qualities and its neuroprotective and anticonvulsant effects are discovered using concentrations of the compound in the millimolar range. This, as well as the low affinity of KYNA at each of the three ionotropic glutamate receptors responsible for these effects [NMDA, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate], together with the realization that KYNA concentrations in the mammalian brain are in the sub-micromolar range, suggested that other receptors might serve as targets of endogenous Kynurenic acid. Kynurenic acid, with a shallower inhibition curve and non-competitively, antagonizes α7nAChRs on cultured hippocampal neurons with an IC50 in the low micromolar range[2]. |
| In Vivo | Kynurenic acid affects the activity of leukocytes in the peripheral blood of mice, although the lowest one (2.5 mg/L) has the most profound influence in contrast to the highest one (250 mg/L), which produces the weakest effect. The lowest Kynurenic acid dose stimulates the proliferative response of T lymphocytes (p<0.05), after 7 and 28 days of administering the acid to the animals[3]. |
| Kinase Assay | CHO-GPR35 stable cells are pretreated with or without pertussis toxin (100 ng/mL) for 16 h before harvesting. Cells are resuspended and homogenized in 10 mM Tris-HCl (pH 7.4), 1 mM EDTA followed by centrifugation at 1000 ×g for 10 min at 4 °C to remove nuclei and cellular debris. Membrane fractions are collected by spinning the supernatant at 38,000 ×g for 30 min and resuspended in 20 mM HEPES (pH 7.5) and 5 mM MgCl2. 25 μg of membranes is incubated at room temperature for 1 h in assay buffer (20 mM HEPES, 5 m MMgCl2, 0.1% bovine serum albumin (pH 7.5)) containing 3 μM GDP and 0.1 nM[35S]GTPγS in the absence or presence of kynurenic acid. Reactions are terminated by vacuum filtration through GF/B filters, and the retained radioactivities are quantified on liquid scintillation counter[1]. |
| Animal Admin | Mouse: The experiment is performed on 160 male BALB/c mice, aged 10-12 weeks, with body weight of 22-26 g. The animals are maintained on a 12-h light/dark cycle at controlled temperature (20 ±1°C) and supplied with rodent chow and water ad libitum throughout the experiment. Mice are divided randomLy into four equal groups: control group (0) not receiving the Kynurenic acid, and three experimental groups administered the Kynurenic acid solution in drinking water at concentrations of 2.5, 25 or 250 mg/L. After 3, 7, 14 and 28 consecutive days of administration of the Kynurenic acid solution, 10 individuals from each group are sacrificed. The animals are anesthetized by inhalation of Aerrane and their blood is collected by heart puncture. Blood collected from five individuals of each group is used for the MTT assay, and from the next five for the flow cytometry[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 358.4±42.0 °C at 760 mmHg |
| Melting Point | 275 °C (dec.)(lit.) |
| Molecular Formula | C10H7NO3 |
| Molecular Weight | 189.167 |
| Flash Point | 170.5±27.9 °C |
| Exact Mass | 189.042587 |
| PSA | 70.42000 |
| LogP | 2.28 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.639 |
| InChIKey | HCZHHEIFKROPDY-UHFFFAOYSA-N |
| SMILES | O=C(O)c1cc(=O)c2ccccc2[nH]1 |
| Storage condition | Store at RT |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UZ9300000 |
| Precursor 8 | |
|---|---|
| DownStream 6 | |
|
Lipocalin 2 modulates the cellular response to amyloid beta.
Cell Death Differ. 21(10) , 1588-99, (2014) The production, accumulation and aggregation of amyloid beta (Aβ) peptides in Alzheimer's disease (AD) are influenced by different modulators. Among these are iron and iron-related proteins, given the... |
|
|
From Memory Impairment to Posttraumatic Stress Disorder-Like Phenotypes: The Critical Role of an Unpredictable Second Traumatic Experience.
J. Neurosci. 35 , 15903-15, (2015) Arousal and stress critically regulate memory formation and retention. Increasing levels of stress produce an inverted U-shaped effect on cognitive performance, including the retention of explicit mem... |
|
|
Metabolite profile analysis reveals association of vitamin B-6 with metabolites related to one-carbon metabolism and tryptophan catabolism but not with biomarkers of inflammation in oral contraceptive users and reveals the effects of oral contraceptives on these processes.
J. Nutr. 145(1) , 87-95, (2015) The use of oral contraceptives (OCs) has been associated with low plasma pyridoxal 5'-phosphate (PLP). The functional consequences are unclear.To determine whether functional vitamin B-6 insufficiency... |
| 4-Hydroxyquinaldic Acid |
| 2-Quinolinecarboxylic acid, 1,4-dihydro-4-oxo- |
| 4-Oxo-1,4-dihydroquinoline-2-carboxylic acid |
| 4-hydroxy-Quinaldic acid |
| 2-Carboxy-4-hydroxyquinoline |
| 1,4-DIHYDRO-4-OXOQUINOLINE-2-CARBOXYLIC ACID |
| 4-Hydroxy-2-quinolincarboxylic acid |
| 4-Hydroxyquinoline-2-carboxylic Acid Hydrate |
| 4-Hydroxyquinoline-2-carboxylic acid |
| 4-Oxo-1,4-dihydro-2-quinolinecarboxylic acid |
| Kynurenic acid |
| EINECS 207-751-5 |
| MFCD00006753 |
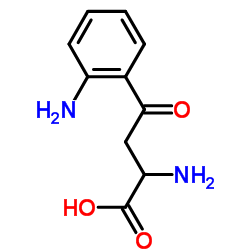 CAS#:343-65-7
CAS#:343-65-7 CAS#:2922-83-0
CAS#:2922-83-0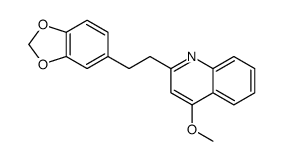 CAS#:529-92-0
CAS#:529-92-0 CAS#:52144-34-0
CAS#:52144-34-0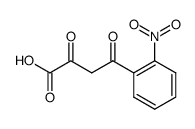 CAS#:446251-68-9
CAS#:446251-68-9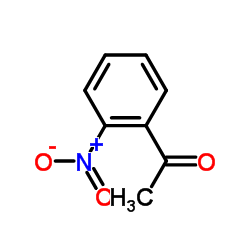 CAS#:577-59-3
CAS#:577-59-3 CAS#:857762-80-2
CAS#:857762-80-2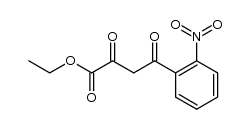 CAS#:178114-28-8
CAS#:178114-28-8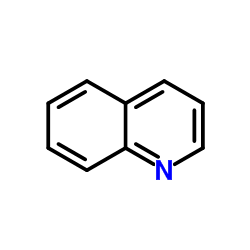 CAS#:91-22-5
CAS#:91-22-5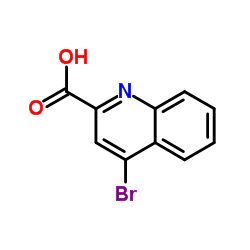 CAS#:209003-46-3
CAS#:209003-46-3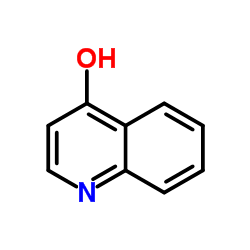 CAS#:529-37-3
CAS#:529-37-3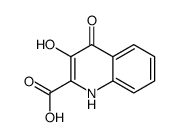 CAS#:33925-79-0
CAS#:33925-79-0 CAS#:168141-99-9
CAS#:168141-99-9
