L-Kynurenine

L-Kynurenine structure
|
Common Name | L-Kynurenine | ||
|---|---|---|---|---|
| CAS Number | 2922-83-0 | Molecular Weight | 208.214 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 466.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C10H12N2O3 | Melting Point | 302.49° C | |
| MSDS | Chinese USA | Flash Point | 236.0±28.7 °C | |
Use of L-KynurenineL-Kynurenine is a metabolite of the amino acid L-tryptophan. L-Kynurenine is an aryl hydrocarbon receptor agonist. |
| Name | L-kynurenine |
|---|---|
| Synonym | More Synonyms |
| Description | L-Kynurenine is a metabolite of the amino acid L-tryptophan. L-Kynurenine is an aryl hydrocarbon receptor agonist. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Kynurenine and its further breakdown products carry out diverse biological functions, including dilating blood vessels during inflammation and regulating the immune response. Some cancers increase kynurenine production, which increases tumor growth. L-kynurenine (Kyn) is an aryl hydrocarbon receptor (AHR) agonist that activates AHR-directed, naive T cell polarization to the anti-inflammatory Treg phenotype. Kynurenine activates AHR signaling at physiological concentrations in H1L7.5c3 cells and acts as an AHR agonist after a 24-hr exposure by inducing the AHR-regulated luciferase gene in H1L7.5c3 mouse hepatocyte cells[1]. |
| In Vivo | Kynurenine dilates arteries from rats as well as humans via Kv7 channels in the vascular smooth muscle. In rats, this tryptophan metabolite causes hypotension, which is partly counteracted by Kv7 channel inhibition[2]. L-kynurenine administered 1 h before the hypoxia-ischemia shows a dose-dependent significant neuroprotective effect, with complete protection at a dose of 300 mg/kg. The induction of c-fos immunoreactivity in cerebral cortex is also blocked by this dose of L-kynurenine[3]. |
| Cell Assay | Luciferase assays are carried out using the H1L7.5c3 cells. At the indicated times (0.5, 2, 4, 6, 12, 18, 24 h) and concentrations (0.1, 1, 10, 100 μM) of exposures to Kynurenine, cells are removed from incubation and allowed to equilibrate to room temperature for 15min. After equilibration, the medium is removed and the cells are washed twice with at room temperature with DPBS. The cells are lysed with 20 µL/well 1× Passive Lysis Buffer and shaken for 20min at room temperature. Luciferase activity is recorded using an Luminometer Microplate Reader[1]. |
| Animal Admin | Rats[3] The effects of increasing doses of L-kynurenine with or without probenecid on concentrations of kynurenic acid in cerebral cortex are examined in 7-day-old rats. Six animals are examined in each group. Animals are treated with L-kynurenine at doses of 100, 200, 300, and 400 mg/kg or kynurenine, 200 mg/kg with probenecid, 50 mg/kg. Animals are killed at 1 h, the brains promptly removed, and the cerebral cortex is dissected and placed in 0.5 mL of chilled 0. 1 M HCl. Kynurenic acid measurements are made by high-performance liquid chromatography with fluorescence detection. Protein measurements are made using a fluorometric assay[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 466.6±45.0 °C at 760 mmHg |
| Melting Point | 302.49° C |
| Molecular Formula | C10H12N2O3 |
| Molecular Weight | 208.214 |
| Flash Point | 236.0±28.7 °C |
| Exact Mass | 208.084793 |
| PSA | 106.41000 |
| LogP | 1.09 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.626 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CY9049700 |
| HS Code | 2922509090 |
|
~% 
L-Kynurenine CAS#:2922-83-0 |
| Literature: BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM; ARMSTRONG, Daniel, W.; PING, Sun; BREITBACH, Zachary, S.; WANG, Chunlei Patent: WO2010/148191 A2, 2010 ; Location in patent: Page/Page column 45-49; 61 ; |
|
~90% 
L-Kynurenine CAS#:2922-83-0 |
| Literature: Jackson, Richard F. W.; Turner, Debra; Block, Michael H. Journal of the Chemical Society - Perkin Transactions 1, 1997 , # 6 p. 865 - 870 |
|
~% 
L-Kynurenine CAS#:2922-83-0 |
| Literature: Lu, Changyuan; Lin, Yu; Yeh, Syun-Ru Biochemistry, 2010 , vol. 49, # 24 p. 5028 - 5034 |
|
~% 
L-Kynurenine CAS#:2922-83-0 |
| Literature: Jackson; Turner; Block Journal of the Chemical Society - Series Chemical Communications, 1995 , # 21 p. 2207 - 2208 |
|
~% 
L-Kynurenine CAS#:2922-83-0 |
| Literature: Jackson, Richard F. W.; Turner, Debra; Block, Michael H. Journal of the Chemical Society - Perkin Transactions 1, 1997 , # 6 p. 865 - 870 |
| Precursor 4 | |
|---|---|
| DownStream 7 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Vitamin B1 deficiency inhibits the increased conversion of tryptophan to nicotinamide in severe food-restricted rats.
Biosci. Biotechnol. Biochem. 79(1) , 103-8, (2015) The conversion of tryptophan (Trp) → nicotinamide (Nam) is an important pathway for supplying vitamin niacin. We reported the following two phenomena: (1) severe food restriction led to an increase in... |
|
|
The urinary ratio of 3-hydroxykynurenine/3-hydroxyanthranilic acid is an index to predicting the adverse effects of D-tryptophan in rats.
J. Nutr. Sci. Vitaminol. 60(4) , 261-8, (2014) The adverse effects of D-tryptophan and the possibility of it being a surrogate index for predicting adverse effects in rats were investigated. Male rats were fed one of several test diets (20% casein... |
|
|
Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver.
J. Pharm. Pharm. Sci. 17(4) , 475-84, (2015) Organic anion-transporting polypeptide (OATP) 1B1 and OATP1B3 contribute to hepatic uptake of numerous drugs. Thus, reduced OATP1B1 and OATP1B3 activity in chronic kidney disease (CKD) may have a majo... |
| (S)-α,2-diamino-γ-oxo-Benzenebutanoic acid |
| (S)-a,2-diamino-g-oxo-Benzenebutanoic acid |
| 3-Anthraniloyl-L-alanine |
| Benzenebutanoic acid, α,2-diamino-γ-oxo-, (αS)- |
| L-3-(o-Aminobenzoyl)alanine |
| 3-anthraniloyl-alanine |
| 3-Anthraniloylalanine |
| Alanine, 3-anthraniloyl-, L- |
| Kynurenine, L- |
| L-KYNURENINE |
| (2S)-2-Amino-4-(2-aminophenyl)-4-oxobutanoic acid |
| (S)-a,2-diamino-γ-oxo-Benzenebutanoic acid |
| Kynurenine |
| Benzenebutanoic acid, α,2-diamino-γ-oxo-, (S)- |
| L-Kynurenin |
| (S)-2-Amino-4-(2-aminophenyl)-4-oxobutanoic acid |
| MFCD00069912 |
| (S)-α,2-Diamino-γ-oxobenzenebutanoic acid |
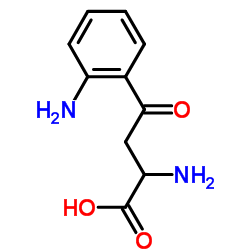
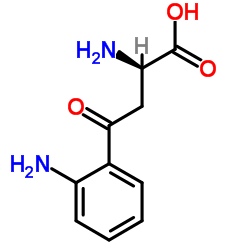



 CAS#:492-27-3
CAS#:492-27-3 CAS#:56-41-7
CAS#:56-41-7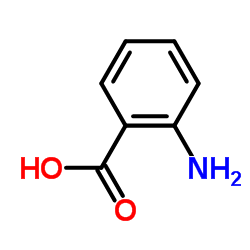 CAS#:118-92-3
CAS#:118-92-3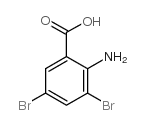 CAS#:609-85-8
CAS#:609-85-8 CAS#:363-36-0
CAS#:363-36-0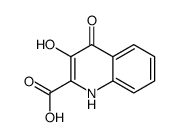 CAS#:33925-79-0
CAS#:33925-79-0 CAS#:15462-45-0
CAS#:15462-45-0
