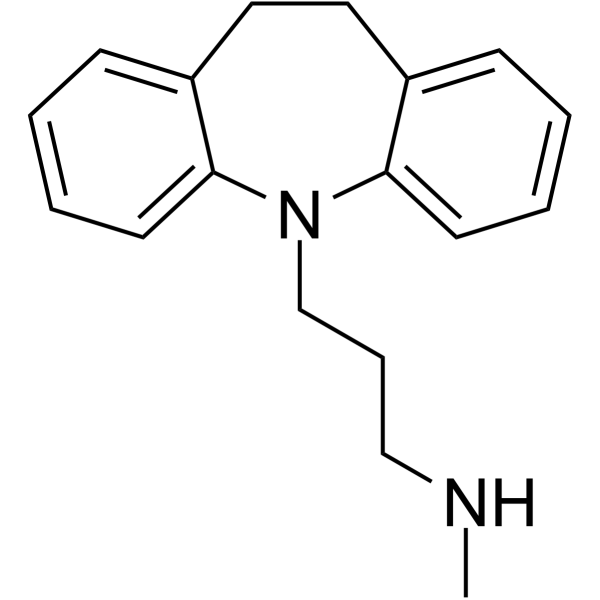
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
HO1575000
-
CHEMICAL NAME :
-
5H-Dibenz(b,f)azepine, 5-(3-(dimethylamino)propyl)-10,11-dihydro-
-
CAS REGISTRY NUMBER :
-
50-49-7
-
BEILSTEIN REFERENCE NO. :
-
0256892
-
LAST UPDATED :
-
199712
-
DATA ITEMS CITED :
-
62
-
MOLECULAR FORMULA :
-
C19-H24-N2
-
MOLECULAR WEIGHT :
-
280.45
-
WISWESSER LINE NOTATION :
-
T C676 BN&T&J B3N1&1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
71428 ug/kg/10D-I
-
TOXIC EFFECTS :
-
Behavioral - rigidity (including catalepsy) Skin and Appendages - sweating Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
150 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - coma Cardiac - other changes Vascular - BP lowering not characterized in autonomic section
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
71 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Cardiac - change in rate Endocrine - changes in luteinizing hormone
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
2 mg/kg/1D
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - other changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
30 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - changes in motor activity (specific assay) Behavioral - coma
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
40 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - changes in motor activity (specific assay) Behavioral - coma
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
3 mg/kg/32H-I
-
TOXIC EFFECTS :
-
Skin and Appendages - photosensitivity (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
30 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - changes in motor activity (specific assay) Behavioral - ataxia
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - child
-
DOSE/DURATION :
-
35 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Cardiac - other changes Lungs, Thorax, or Respiration - dyspnea
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
450 mg/kg
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, irritative (after systemic exposure)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
40 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
5357 ug/kg/5D-I
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - changes in REM sleep (human)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
8 mg/kg/3D-I
-
TOXIC EFFECTS :
-
Behavioral - excitement
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - changes in motor activity (specific assay) Nutritional and Gross Metabolic - body temperature decrease
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
79 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
9300 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
188 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - changes in motor activity (specific assay) Nutritional and Gross Metabolic - body temperature decrease
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
51600 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
195 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - wakefulness Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
21 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - parasympatholytic Behavioral - wakefulness Behavioral - changes in motor activity (specific assay)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
107 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - smooth muscle relaxant (mechanism undefined, spasmolytic)
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
45 mg/kg
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - flaccid paralysis without anesthesia (usually neuromuscular blockage) Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - respiratory depression
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
20 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
385 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
18 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
350 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - anticonvulsant
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Mammal - species unspecified
-
DOSE/DURATION :
-
455 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3600 mg/kg/4W-I
-
TOXIC EFFECTS :
-
Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5760 mg/kg/22W-I
-
TOXIC EFFECTS :
-
Cardiac - EKG changes not diagnostic of specified effects Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
75 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating female 60 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
378 mg/kg
-
SEX/DURATION :
-
lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - behavioral Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
165 mg/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating female 1-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 14-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
210 mg/kg
-
SEX/DURATION :
-
female 21 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 18-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - biochemical and metabolic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
130 mg/kg
-
SEX/DURATION :
-
female 8-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
65 mg/kg
-
SEX/DURATION :
-
female 8-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - behavioral
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
175 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
87500 ug/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
188 mg/kg
-
SEX/DURATION :
-
male 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraplacental
-
DOSE :
-
80 ug/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraplacental
-
DOSE :
-
80 ug/kg
-
SEX/DURATION :
-
female 15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
390 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
55 mg/kg
-
SEX/DURATION :
-
female 6-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
255 mg/kg
-
SEX/DURATION :
-
female 1-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
188 mg/kg/5D (Intermittent)
-
REFERENCE :
-
IMSCE2 IRCS Medical Science. (Lancaster, UK) V.12-14, 1984-86. For publisher information, see MSCREJ. Volume(issue)/page/year: 14,1129,1986 *** REVIEWS *** TOXICOLOGY REVIEW DICPBB Drug Intelligence and Clinical Pharmacy. (POB 42435, Cincinnati, OH 45242) V.3- 1969- Volume(issue)/page/year: 8,690,1974 TOXICOLOGY REVIEW CRTXB2 CRC Critical Reviews in Toxicology. (CRC Press, Inc., 2000 Corporate Blvd., NW, Boca Raton, FL 33431) V.1- 1971- Volume(issue)/page/year: 1(1),93,1971 TOXICOLOGY REVIEW CLCHAU Clinical Chemistry (Winston-Salem, NC). (American Assoc. for Clinical Chemistry, 1725 K St., NW, Washington, DC 20006) V.1- 1955- Volume(issue)/page/year: 19,361,1973 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW ADVPA3 Advances in Pharmacology. (New York, NY) V.1-6, 1962-68. For publisher information, see AVPCAQ. Volume(issue)/page/year: 4,263,1966 TOXICOLOGY REVIEW FNSCA6 Forensic Science. (Lausanne, Switzerland) V.1-11, 1972-78. For pub lisher information, see FSINDR. Volume(issue)/page/year: 2,67,1973 TOXICOLOGY REVIEW CTOXAO Clinical Toxicology. (New York, NY) V.1-18, 1968-81. For publisher information, see JTCTDW. Volume(issue)/page/year: 2,403,1969 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4727 No. of Facilities: 7 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 103 (estimated) No. of Female Employees: 52 (estimated)
|
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine Structure](https://www.chemsrc.com/caspic/389/50-49-7.png)
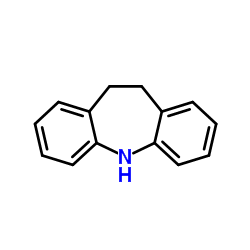
![3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine oxide structure](https://www.chemsrc.com/caspic/457/6829-98-7.png)

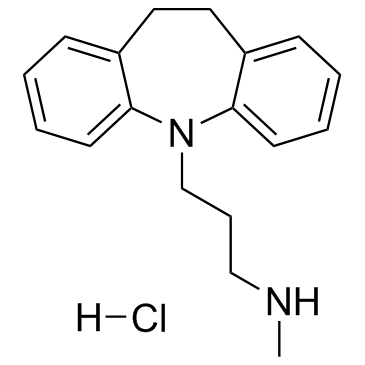
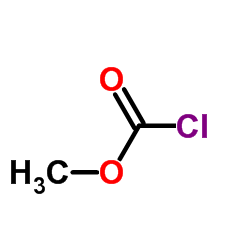
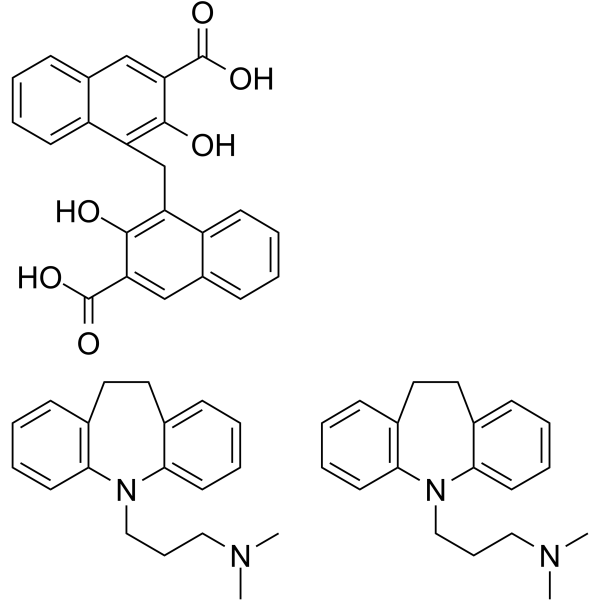 CAS#:10075-24-8
CAS#:10075-24-8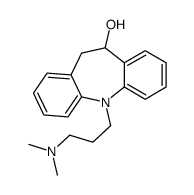 CAS#:796-28-1
CAS#:796-28-1![11-[3-(dimethylamino)propyl]-5,6-dihydrobenzo[b][1]benzazepin-3-ol structure](https://www.chemsrc.com/caspic/327/303-70-8.png) CAS#:303-70-8
CAS#:303-70-8