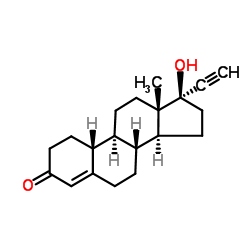
19-Norethindrone acetate

19-Norethindrone acetate structure
|
Common Name | 19-Norethindrone acetate | ||
|---|---|---|---|---|
| CAS Number | 51-98-9 | Molecular Weight | 340.456 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 454.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C22H28O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 197.1±28.8 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of 19-Norethindrone acetateNorethindrone acetate is a female progestin approved by FDA for the treatment of endometriosis, uterine bleeding caused by abnormal hormone levels, and secondary amenorrhea. |
| Name | norethisterone acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Norethindrone acetate is a female progestin approved by FDA for the treatment of endometriosis, uterine bleeding caused by abnormal hormone levels, and secondary amenorrhea. |
|---|---|
| Related Catalog | |
| Target |
Progesterone Receptor[1] |
| In Vivo | Norethindrone acetate could be a cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis. Subjects treated with norethindrone acetate obtain dysmenorrhea and noncyclic pelvic pain relief[1]. Norethindrone acetate alone is a well-tolerated, effective option to manage pain and bleeding for all stages of endometriosis. Post-Norethindrone acetate bleeding scores are improved regardless of prior hormonal regimen, and post-Norethindrone acetate pain scores improved in all patients except for those previously prescribed GnRH-agonist plus add-back[2]. Norethindrone acetate shows low acute toxicity in experimental animals and is consistent with the lack of toxicity seen in humans. Administration of norethindrone acetate alone to rodents at several multiples of the human dose results in no treatment related mortality, hematological changes, behavioral changes, or organ pathology[3]. Norethindrone acetate administration leads to significant and proportional reductions of the concentrations of triglycerides, cholesterol and phospholipids of plasma lipoproteins of density <1.006 of rats fed a high carbohydrate diet. Norethindrone acetate (0.1 mM) also significantly inhibits the incorporation of both palmitate and glycerol into triglycerides of isolated hepatocytes from fed rats[4]. |
| Animal Admin | Rats: Female Sprague-Dawley rats (200-210 g), 6 of which serve as controls, are individually caged in an animal room illuminated from 9:00 a.m. to 9:00 p.m. Each of the 7 rats receiving norethindrone acetate is fed 35 μg/day for 2 weeks. Water and the rat chow which is high in carbohydrate are available ad libitum[4]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 454.7±45.0 °C at 760 mmHg |
| Molecular Formula | C22H28O3 |
| Molecular Weight | 340.456 |
| Flash Point | 197.1±28.8 °C |
| Exact Mass | 340.203857 |
| PSA | 43.37000 |
| LogP | 3.99 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.556 |
| Storage condition | 2~8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R40;R48 |
| Safety Phrases | S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | RC8965000 |
| HS Code | 2915299090 |
|
~96% 
19-Norethindron... CAS#:51-98-9 |
| Literature: Leung; Karunanithy; Becket; Yeo Steroids, 1985 , vol. 46, # 1 p. 639 - 647 |
|
~87% 
19-Norethindron... CAS#:51-98-9 |
| Literature: Zhou, Dong; Carlson, Kathryn E.; Katzenellenbogen, John A.; Welch, Michael J. Journal of Medicinal Chemistry, 2006 , vol. 49, # 15 p. 4737 - 4744 |
|
~% 
19-Norethindron... CAS#:51-98-9 |
| Literature: Iriarte et al. Journal of the American Chemical Society, 1959 , vol. 81, p. 436 |
|
~% 
19-Norethindron... CAS#:51-98-9 |
| Literature: Iriarte et al. Journal of the American Chemical Society, 1959 , vol. 81, p. 436 |
| HS Code | 2915299090 |
|---|---|
| Summary | 2915299090 salts of acetic acid。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:5.5%。general tariff:50.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Development of a phospholipidosis database and predictive quantitative structure-activity relationship (QSAR) models.
Toxicol. Mech. Methods 18 , 217-27, (2008) ABSTRACT Drug-induced phospholipidosis (PL) is a condition characterized by the accumulation of phospholipids and drug in lysosomes, and is found in a variety of tissue types. PL is frequently manifes... |
|
|
Molecular modulation of estrogen-induced apoptosis by synthetic progestins in hormone replacement therapy: an insight into the women's health initiative study.
Cancer Res. 74(23) , 7060-8, (2014) Hormone replacement therapy (HRT) is widely used to manage menopausal symptoms in women and can be comprised of an estrogen alone or an estrogen combined with a progestin. The Women's Health Initiativ... |
| 17α-Ethinyl-19-nortestosterone acetate |
| NORLUTATE |
| 17α-Ethynyl-19-nortestosterone acetate |
| Estr-4-en-3-one, 17-(acetyloxy)-17-ethynyl-, (17β)- |
| 19-Norethindrone acetate |
| 17β-Acetoxy-19-nor-17α-pregn-4-en-20-yn-3-one |
| (17α)-3-Oxo-19-norpregn-4-en-20-yn-17-yl acetate |
| 19-Nor-17α-pregn-4-en-20-yn-3-one, 17-hydroxy-, acetate |
| 17-α-Ethynyl-19-nortestosterone acetate |
| 17α-Ethinyl-19-nortestosterone-17β-acetate |
| Milligynon |
| MFCD04039850 |
| 19-Norpregn-4-en-20-yn-3-one, 17-(acetyloxy)-, (17α)- |
| 17α-Ethynyl-17β-acetoxy-19-norandrost-4-en-3-one |
| [(8R,9S,10R,13S,14S,17R)-17-ethynyl-13-methyl-3-oxo-1,2,6,7,8,9,10,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-yl] acetate |
| 17-Acetoxy-19-nor-17α-pregn-4-en-20-yn-3-one |
| Norethindrone acetate |
| 19-Norpregn-4-en-20-yn-3-one, 17- (acetyloxy)-, (17α)- |
| Norethisterone acetate |
| Primolut-Nor |
| 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate |
| EINECS 200-132-0 |
| Norlutin A |
| 17-α-Ethinyl-19-nortestosterone-17-β-acetate |
| Norlutin acetate |
| 17α-Ethynyl-17-hydroxyestr-4-en-3-one acetate |
| Norethindrone 17-acetate |
| 17β-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate |
| 19-nor-17α-Pregn-4-en-20-yn-3-one, 17-acetoxy- |
| 19-Nor-17α-ethynyltestosterone Acetate |
| Estr-4-en-3-one, 17α-ethynyl-17-hydroxy-, acetate |
| 19-Nor-17α-pregn-4-en-20-yn-3-one, 17-hydroxy-, acetate (8CI) |
| Aygestin |
| (8R,9S,10R,13S,14S,17R)-17-ethynyl-13-methyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl acetate |
| Norethisteron acetate |
| Norlutin-A |


 CAS#:472-54-8
CAS#:472-54-8