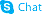Furosemide
Furosemide structure
|
Common Name | Furosemide | ||
|---|---|---|---|---|
| CAS Number | 54-31-9 | Molecular Weight | 330.744 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 582.1±60.0 °C at 760 mmHg | |
| Molecular Formula | C12H11ClN2O5S | Melting Point | 220 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 305.9±32.9 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of FurosemideFurosemide (Lasix) is a loop diuretic inhibitor of Na+/2Cl-/K+ (NKCC) cotransporter of which used in the treatment of congestive heart failure and edema.Target: NKCC Furosemide (INN/BAN) or frusemide is a loop diuretic used in the treatment of congestive heart failure and edema. It is most commonly marketed by Sanofi under the brand name Lasix, and also under the brand names Fusid and Frumex. It has also been used to prevent Thoroughbred and Standardbred race horses from bleeding through the nose during races.Along with some other diuretics, furosemide is also included on the World Anti-Doping Agency's banned drug list due to its alleged use as a masking agent for other drugs.Furosemide, like other loop diuretics, acts by inhibiting NKCC2, the luminal Na-K-2Cl symporter in the thick ascending limb of the loop of Henle. The action on the distal tubules is independent of any inhibitory effect on carbonic anhydrase or aldosterone; it also abolishes the corticomedullary osmotic gradient and blocks negative, as well as positive, free water clearance.Because of the large NaCl absorptive capacity of the loop of Henle, diuresis is not limited by development of acidosis, as it is with the carbonic anhydrase inhibitors. Additionally, furosemide is a noncompetitive subtype-specific blocker of GABA-A receptors. Furosemide has been reported to reversibly antagonize GABA-evoked currents of α6β2γ2 receptors at uM concentrations, but not α1β2γ2 receptors. During development, the α6β2γ2 receptor increases in expression in cerebellar granule neurons, corresponding to increased sensitivity to furosemide |
| Name | furosemide |
|---|---|
| Synonym | More Synonyms |
| Description | Furosemide (Lasix) is a loop diuretic inhibitor of Na+/2Cl-/K+ (NKCC) cotransporter of which used in the treatment of congestive heart failure and edema.Target: NKCC Furosemide (INN/BAN) or frusemide is a loop diuretic used in the treatment of congestive heart failure and edema. It is most commonly marketed by Sanofi under the brand name Lasix, and also under the brand names Fusid and Frumex. It has also been used to prevent Thoroughbred and Standardbred race horses from bleeding through the nose during races.Along with some other diuretics, furosemide is also included on the World Anti-Doping Agency's banned drug list due to its alleged use as a masking agent for other drugs.Furosemide, like other loop diuretics, acts by inhibiting NKCC2, the luminal Na-K-2Cl symporter in the thick ascending limb of the loop of Henle. The action on the distal tubules is independent of any inhibitory effect on carbonic anhydrase or aldosterone; it also abolishes the corticomedullary osmotic gradient and blocks negative, as well as positive, free water clearance.Because of the large NaCl absorptive capacity of the loop of Henle, diuresis is not limited by development of acidosis, as it is with the carbonic anhydrase inhibitors. Additionally, furosemide is a noncompetitive subtype-specific blocker of GABA-A receptors. Furosemide has been reported to reversibly antagonize GABA-evoked currents of α6β2γ2 receptors at uM concentrations, but not α1β2γ2 receptors. During development, the α6β2γ2 receptor increases in expression in cerebellar granule neurons, corresponding to increased sensitivity to furosemide |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 582.1±60.0 °C at 760 mmHg |
| Melting Point | 220 °C (dec.)(lit.) |
| Molecular Formula | C12H11ClN2O5S |
| Molecular Weight | 330.744 |
| Flash Point | 305.9±32.9 °C |
| Exact Mass | 330.007721 |
| PSA | 131.01000 |
| LogP | 3.10 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.658 |
| Stability | Stable, but light sensitive, air sensitive and hygroscopic. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H360 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R61 |
| Safety Phrases | S7-S16-S36/37-S45-S53-S36/37/39-S22 |
| RIDADR | UN 1230 3/PG 2 |
| WGK Germany | 3 |
| RTECS | CB2625000 |
| HS Code | 2942000000 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Revival of physostigmine - a novel HPLC assay for simultaneous determination of physostigmine and its metabolite eseroline designed for a pharmacokinetic study of septic patients.
Clin. Chem. Lab Med. 53 , 1259-64, (2015) Physostigmine, commonly used as an antidote in anticholinergic poisoning, is reported to have additional pharmacological effects, such as activation of the cholinergic anti-inflammatory pathway in sep... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| Mirfat |
| furesis |
| Odemase |
| Frumex |
| furosedon |
| Durafurid |
| Furosemide,5-(Aminosulfonyl)-4-chloro-2-([2-furanylmethyl]amino)benzoicacid |
| Frusemide |
| frusid |
| dryptal |
| Benzoic acid, 5-(aminosulfonyl)-4-chloro-2-[(2-furanylmethyl)amino]- |
| Frusetic |
| Errolon |
| trofurit |
| Nicorol |
| EINECS 200-203-6 |
| Urex |
| macasirool |
| lasilix |
| Furosemid |
| Oedemex |
| Discoid |
| katlex |
| Impugan |
| profemin |
| desdemin |
| Furosemide |
| Fusid |
| 4-Chloro-2-[(2-furylmethyl)amino]-5-sulfamoylbenzoic acid |
| Lasix |
| Eutensin |
| fulsix |
| rosemide |
| MFCD00010549 |
| Fuluvamide |
| 4-Chloro-N-Furfuryl-5-Sulfamoylanthranilic Acid |
 CAS#:396-01-0
CAS#:396-01-0 CAS#:78846-76-1
CAS#:78846-76-1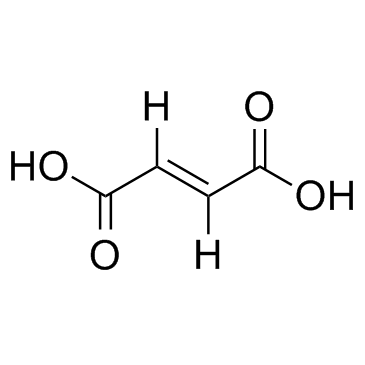 CAS#:110-17-8
CAS#:110-17-8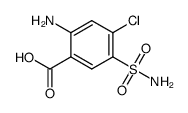 CAS#:3086-91-7
CAS#:3086-91-7![4-chloro-2-[(2,5-dimethoxy-2,5-dihydro-furan-2-ylmethyl)-amino]-5-sulfamoyl-benzoic acid structure](https://www.chemsrc.com/caspic/316/1351169-05-5.png) CAS#:1351169-05-5
CAS#:1351169-05-5