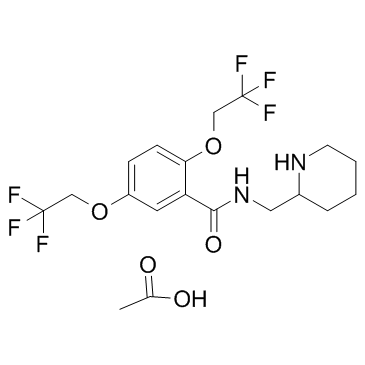
flecainide acetate
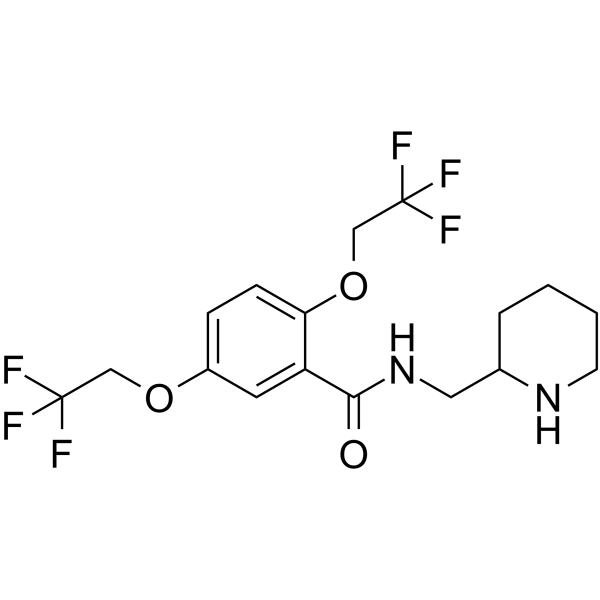
flecainide acetate structure
|
Common Name | flecainide acetate | ||
|---|---|---|---|---|
| CAS Number | 54143-56-5 | Molecular Weight | 474.395 | |
| Density | 1.286g/cm3 | Boiling Point | 434.9ºC at 760mmHg | |
| Molecular Formula | C19H24F6N2O5 | Melting Point | 145-147℃ | |
| MSDS | Chinese USA | Flash Point | 216.8ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of flecainide acetateFlecainide(Tambocor) is a class 1C antiarrhythmic drug especially used for the management of supraventricular arrhythmia; works by blocking the Nav1.5 sodium channel in the heart, causing prolongation of the cardiac action potential.IC50 Value:Target: Nav1.5 channelFlecainide is a class 1C antiarrhythmic drug especially used for the management of supraventricular arrhythmia. Flecainide works by blocking the Nav1.5 sodium channel in the heart, causing prolongation of the cardiac action potential.in vitro: Under the current-clamp condition, flecainide (1-100 microM) prolonged the action potential duration at both the early and the late phases of repolarization in a concentration-dependent manner without affecting the resting membrane potential [1]. At a holding potential (HP) of -120 mV, flecainide use-dependently blocked WT and G1306E I(Na) equally but was more potent on R1448C channels. For WT, the extent of block depended on a holding voltage more negative than the activation threshold, being greater at -90 mV as compared to -120 and -180 mV [2].in vivo: Flecainide (80-130 mg/m(2) orally) resulted in termination of the tachycardia in all 8 patients. Acute pharmacological termination of arrhythmia occurred with oral flecainide loading in 1 and temporarily with intravenous esmolol loading in 1 patient. Adjuvant therapy in form of propranolol was used in 5 and digoxin in 2 [3].Clinical trial: To Evaluate the Impact of Oral Flecainide on Quality of Life in Patients With Paroxysmal Atrial Fibrillation . Phase4 |
| Name | flecainide acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Flecainide(Tambocor) is a class 1C antiarrhythmic drug especially used for the management of supraventricular arrhythmia; works by blocking the Nav1.5 sodium channel in the heart, causing prolongation of the cardiac action potential.IC50 Value:Target: Nav1.5 channelFlecainide is a class 1C antiarrhythmic drug especially used for the management of supraventricular arrhythmia. Flecainide works by blocking the Nav1.5 sodium channel in the heart, causing prolongation of the cardiac action potential.in vitro: Under the current-clamp condition, flecainide (1-100 microM) prolonged the action potential duration at both the early and the late phases of repolarization in a concentration-dependent manner without affecting the resting membrane potential [1]. At a holding potential (HP) of -120 mV, flecainide use-dependently blocked WT and G1306E I(Na) equally but was more potent on R1448C channels. For WT, the extent of block depended on a holding voltage more negative than the activation threshold, being greater at -90 mV as compared to -120 and -180 mV [2].in vivo: Flecainide (80-130 mg/m(2) orally) resulted in termination of the tachycardia in all 8 patients. Acute pharmacological termination of arrhythmia occurred with oral flecainide loading in 1 and temporarily with intravenous esmolol loading in 1 patient. Adjuvant therapy in form of propranolol was used in 5 and digoxin in 2 [3].Clinical trial: To Evaluate the Impact of Oral Flecainide on Quality of Life in Patients With Paroxysmal Atrial Fibrillation . Phase4 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.286g/cm3 |
|---|---|
| Boiling Point | 434.9ºC at 760mmHg |
| Melting Point | 145-147℃ |
| Molecular Formula | C19H24F6N2O5 |
| Molecular Weight | 474.395 |
| Flash Point | 216.8ºC |
| Exact Mass | 474.158936 |
| PSA | 96.89000 |
| LogP | 4.25130 |
| Storage condition | 2-8°C |
| Water Solubility | Soluble in water and in anhydrous ethanol. It is freely soluble in dilute acetic acid and practically insoluble in dilute hydrochloric acid. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H335-H360 |
| Precautionary Statements | P201-P280-P301 + P312 + P330-P305 + P351 + P338-P308 + P313 |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | 22-26-36/37/39-45 |
| RIDADR | UN 3249 |
| WGK Germany | - |
| RTECS | CV5792550 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~87% 
flecainide acetate CAS#:54143-56-5 |
| Literature: "Joint Stock Company Grindeks" Patent: EP1918280 A1, 2008 ; Location in patent: Page/Page column 4; 8 ; |
|
~87% 
flecainide acetate CAS#:54143-56-5 |
| Literature: Geneva Pharmaceuticals, Inc. Patent: US6458957 B1, 2002 ; |
|
~87% 
flecainide acetate CAS#:54143-56-5 |
| Literature: Wang, Zhi-Xian; Li, Yuanqiang; Guntoori, Bhaskar Reddy Patent: US2005/59825 A1, 2005 ; Location in patent: Page/Page column 4 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
|
Multiple modes of ryanodine receptor 2 inhibition by flecainide.
Mol. Pharmacol. 86(6) , 696-706, (2014) Catecholaminergic polymorphic ventricular tachycardia (CPVT) causes sudden cardiac death due to mutations in cardiac ryanodine receptors (RyR2), calsequestrin, or calmodulin. Flecainide, a class I ant... |
|
|
Serum flecainide S/R ratio reflects the CYP2D6 genotype and changes in CYP2D6 activity.
Drug Metab. Pharmacokinet. 30 , 257-62, (2015) The aims of this study were to clarify whether the ratio of S- to R-flecainide (S/R ratio) in the serum flecainide concentration was associated with the stereoselectivity of flecainide metabolism, and... |
|
|
A misguided 'pill in the pocket' approach with flecainide leading to cardiac arrest.
BMJ Case Rep. 2012 , doi:10.1136/bcr-2012-006868, (2012)
|
| FLECAINIDE MONOACETATE |
| acetic acid,N-(piperidin-2-ylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide |
| R 818 |
| Flecainide acetate salt |
| acide acétique - N-(pipéridin-2-ylméthyl)-2,5-bis(2,2,2-trifluoroéthoxy)benzamide (1:1) |
| N-(Piperidin-2-ylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide acetate (1:1) |
| EINECS 258-997-5 |
| Flecainide acetate |
| N-(2-Piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide acetate (1:1) |
| N-(2-Piperidylmethyl)-2,5-bis-(2,2,2-trifluoroethoxy)benzamide acetate salt |
| UNII:M8U465Q1WQ |
| N-(piperidin-2-ylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide acetate |
| Benzamide, N-(2-piperidinylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)-, acetate (1:1) |
| N-(Piperidin-2-ylmethyl)-2,5-bis(2,2,2-trifluorethoxy)benzolcarboxamidacetat |
| Flecainide (acetate) |